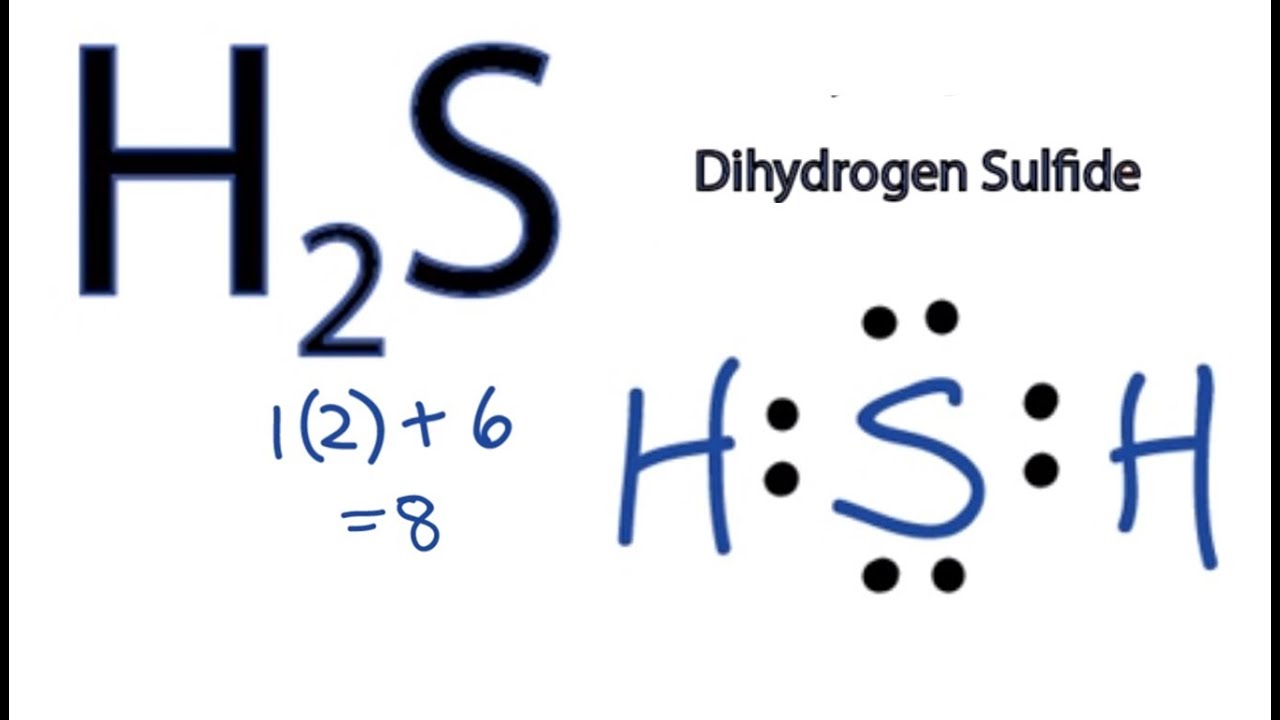
Estructura De Lewis H2s Estudiar
The H2S Lewis structure represents the arrangement of atoms and electrons in the molecule. To draw the H2S Lewis structure, we start by identifying the valence electrons of hydrogen and sulfur atoms. Then, we place the atoms in a way that satisfies the octet rule, where each atom has eight electrons in its valence shell.
H2S at emaze Presentation
Step #1: Calculate the total number of valence electrons. Here, the given molecule is H2S (dihydrogen sulfide). In order to draw the lewis structure of H2S, first of all you have to find the total number of valence electrons present in the H2S molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).

Hydrogen sulfide h2s molecule skeletal formula Vector Image
A step-by-step explanation of how to write the Lewis Dot Structure for H2S (Dihydrogen Sulfide).The H2S Lewis structure is similar to the structure for water.

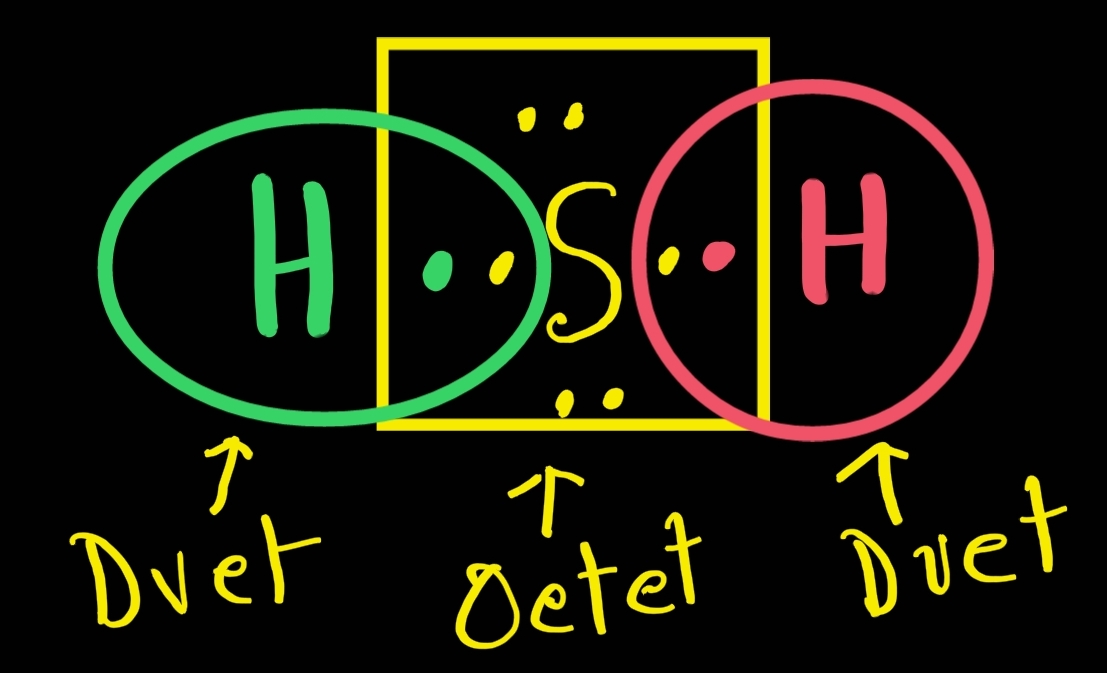
So far, we’ve used 8 of the H2S Lewis structure’s total 8 outermost valence shell electrons. Two
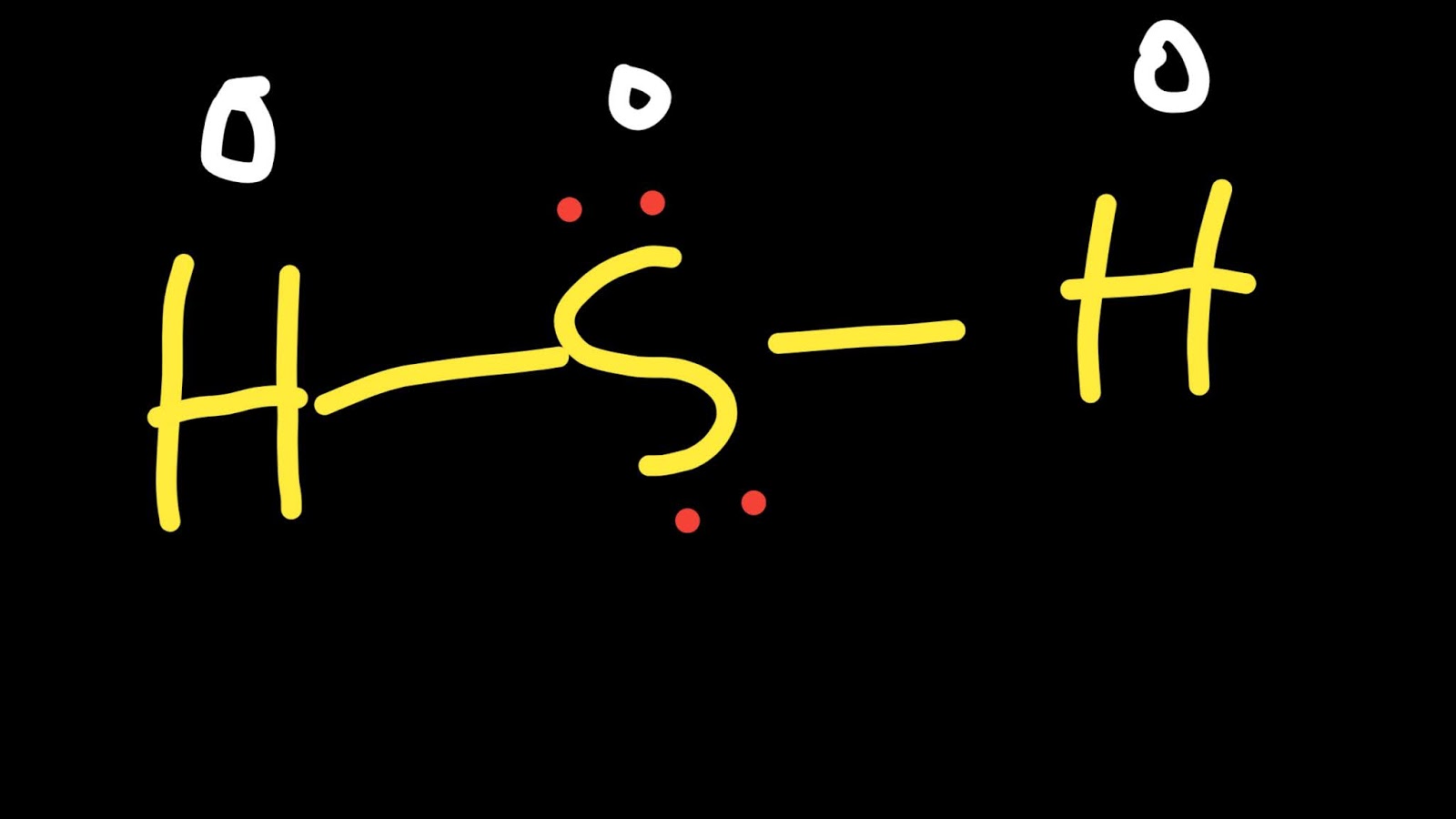
The Lewis Structure of Hydrogen Sulfide is easy to draw and understand. In this compound, both the hydrogen atoms require one electron to make the covalent bond with Sulfur. The Lewis structure of H2S is similar to H 2 S. Sulfur needs eight electrons to fulfill the requirements for Octet Rule. But Hydrogen only requires a single electron to.
【4 Steps】H2S Lewis StructureLewis Structure for H2S (Dihydrogen Sulfide)Lewis Dot Structure
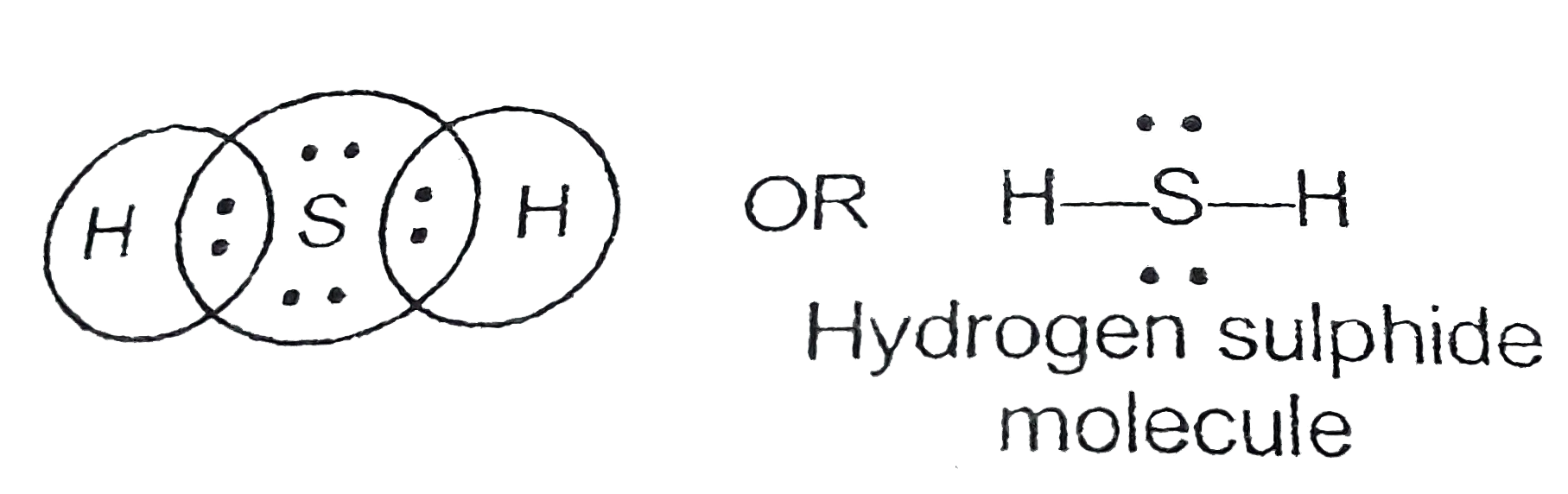
Hydrogen and sulfur are both non-metals, and so they SHARE electrons to form covalent bonds (this makes it a covalent aka MOLECULAR compound). Sulfur needs t.
31+ H2S Lewis Structure Pictures Bepe Enthusiastic
Hydrogen sulfide (H2S) consists of two hydrogen (H) atoms and one sulfur (S) atom. Sulfur is located in group 16 of the periodic table, indicating that it has six valence electrons, while hydrogen belongs to group 1 and brings one valence electron per atom. Step-by-Step Guide to Drawing the Lewis Structure of H2S 1. Determining
Lewis Structure, Molecular Shape and Hybridization for H2S YouTube
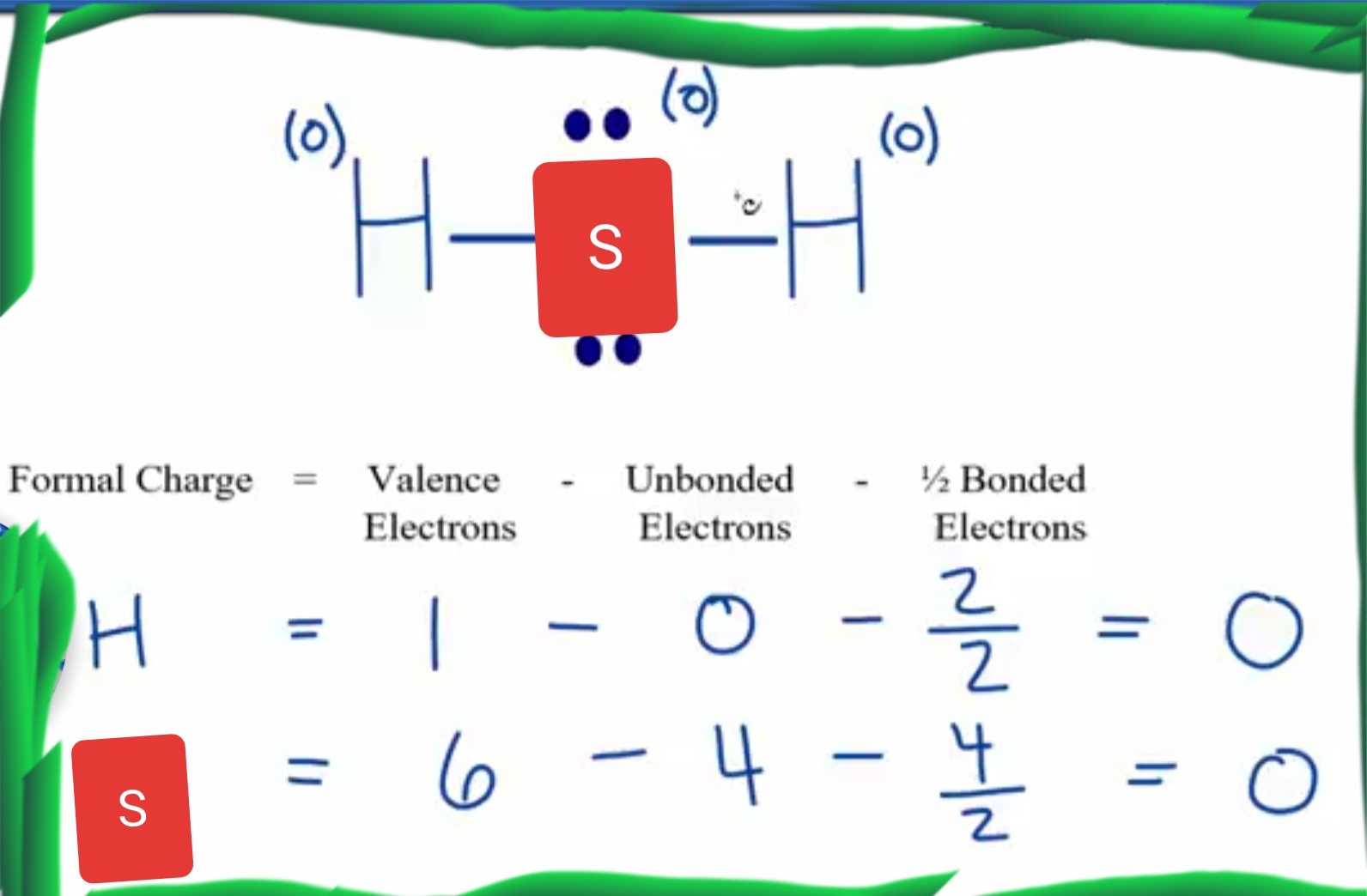
To properly draw the H 2 S Lewis structure, follow these steps: #1 Draw a rough sketch of the structure. #2 Next, indicate lone pairs on the atoms. #3 Indicate formal charges on the atoms, if necessary. Let's break down each step in more detail.
H2S Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar,Octet Rule
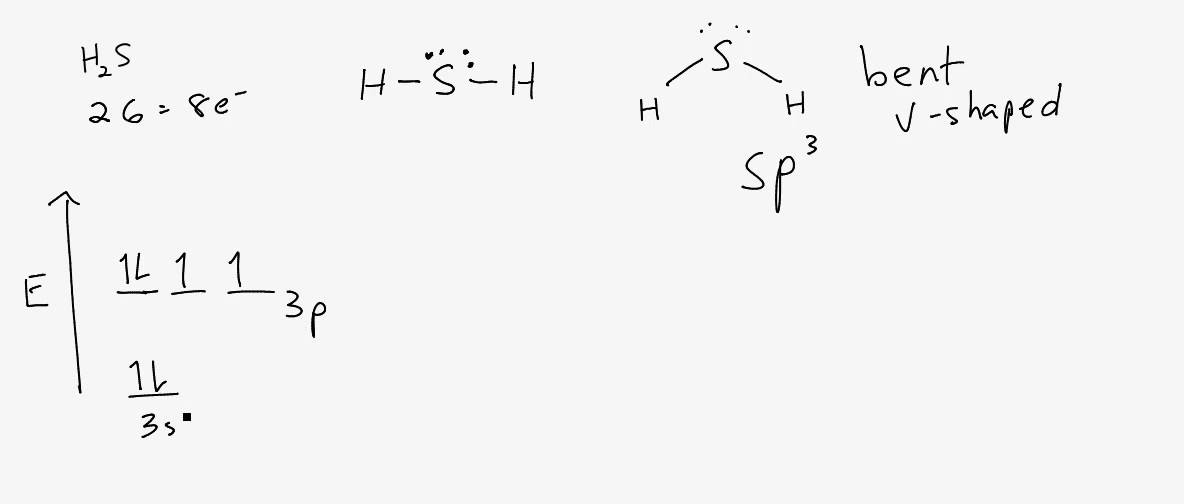
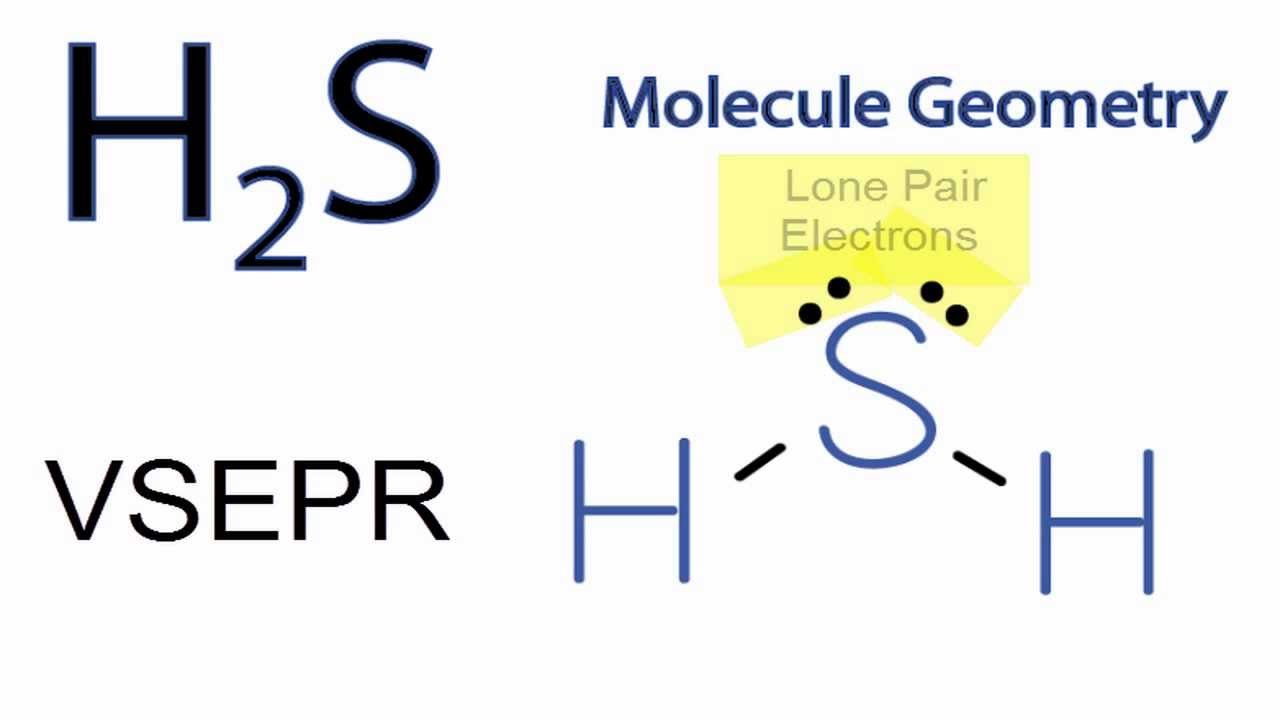
Struktur H2S Lewis memiliki atom sulfur (S) di tengahnya yang dikelilingi oleh dua atom hidrogen (H). Terdapat 2 ikatan tunggal antara atom belerang (S) dan masing-masing atom hidrogen (H). Terdapat 2 pasangan elektron bebas pada atom belerang (S). Jika Anda tidak memahami apa pun dari gambar struktur Lewis H2S di atas, tetaplah bersama saya.
H2S Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar,Octet Rule
What is the Lewis structure of hydrogen sulfide H 2 S?. Hydrogen sulfide H 2 S is a gas with a foul smell, often described as being similar to rotten eggs. It is composed of two hydrogen atoms and one sulfur atom, and is important in various industrial processes and biochemical reactions. To draw the Lewis structure of hydrogen sulfide, follow these step-by-step instructions.

Lewis Structure Hydrogen Sulfide H2s Stock Vector (Royalty Free) 2264370065 Shutterstock
Lewis structures - also called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures ( LEDs ) - are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. [1] [2] [3] A Lewis structure can be drawn for any covalently.
H2S Lewis Structure, Molecular Geometry, Hybridization and Polarity
The molar mass of H2S is 34.08 g/mol and its density is 1.363 g dm-3. The melting point and boiling point of H2S are -82℃ and -60℃ respectively. H2S has a covalent bond because the sulfur atom completes its octet by sharing 2 electrons with 2 hydrogen atoms and thus forms a covalent bond.

H2S Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar,Octet Rule
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.
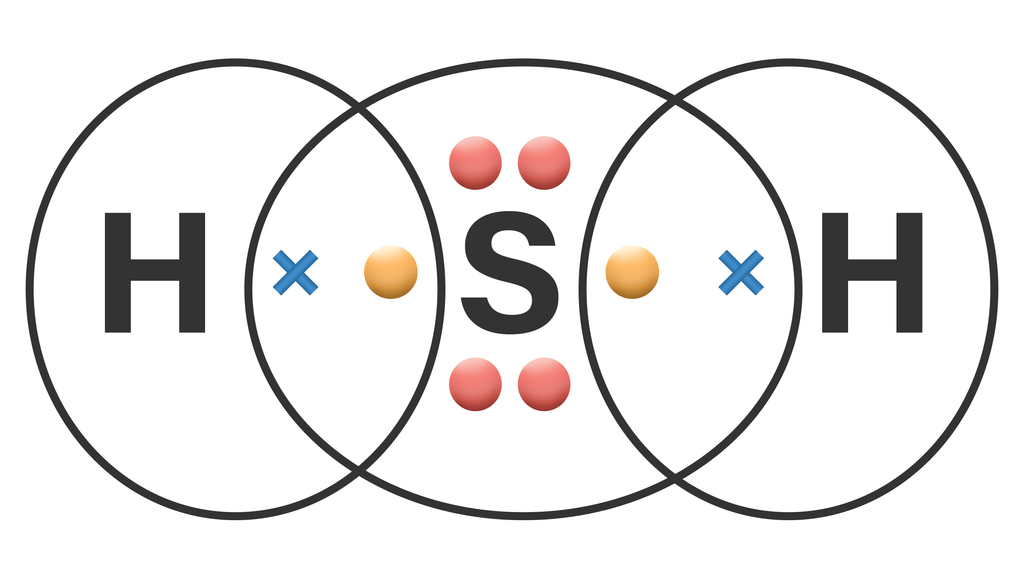
Gambarkan struktur Lewis dari H2S...
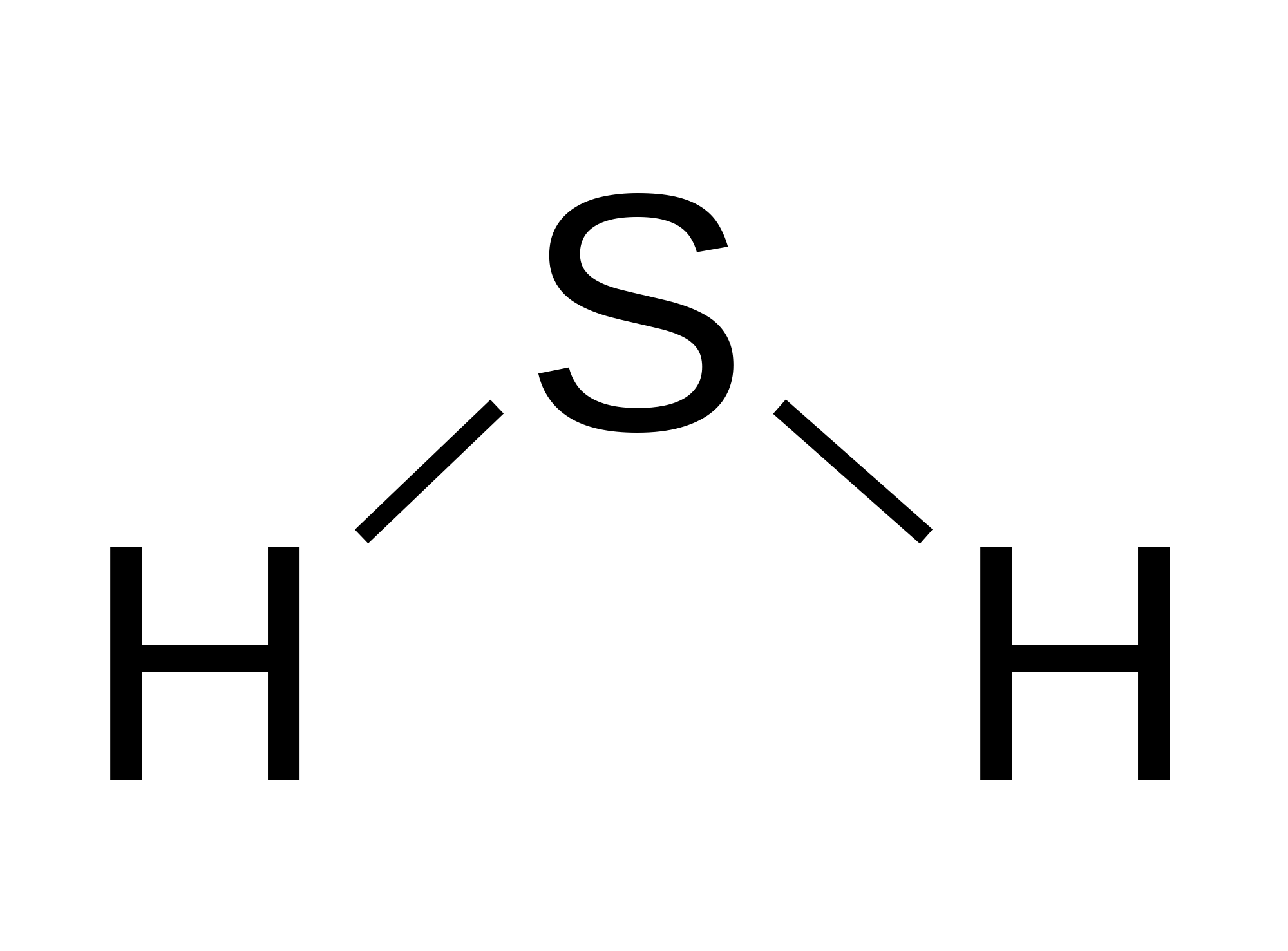
The Lewis structure of hydrogen sulfide is best represented as a bent H{eq}_2 {/eq}S molecule with two lone pairs of electrons on the S atom represented by two pairs of dots (or two bars).

How to draw H2S Lewis Structure? Science Education and Tutorials
Lewis structure of Hydrogen sulfide (H 2 S) contains two S-H single bonds around sulfur atom. Also, there are two lone pairs around sulfur atom. Concept of number of total valence electrons of sulfur and hydrogen atoms are used to draw lewis structure of H 2 S. Each step of drawing lewis structure of H 2 S is explained in detail in this tutorial.
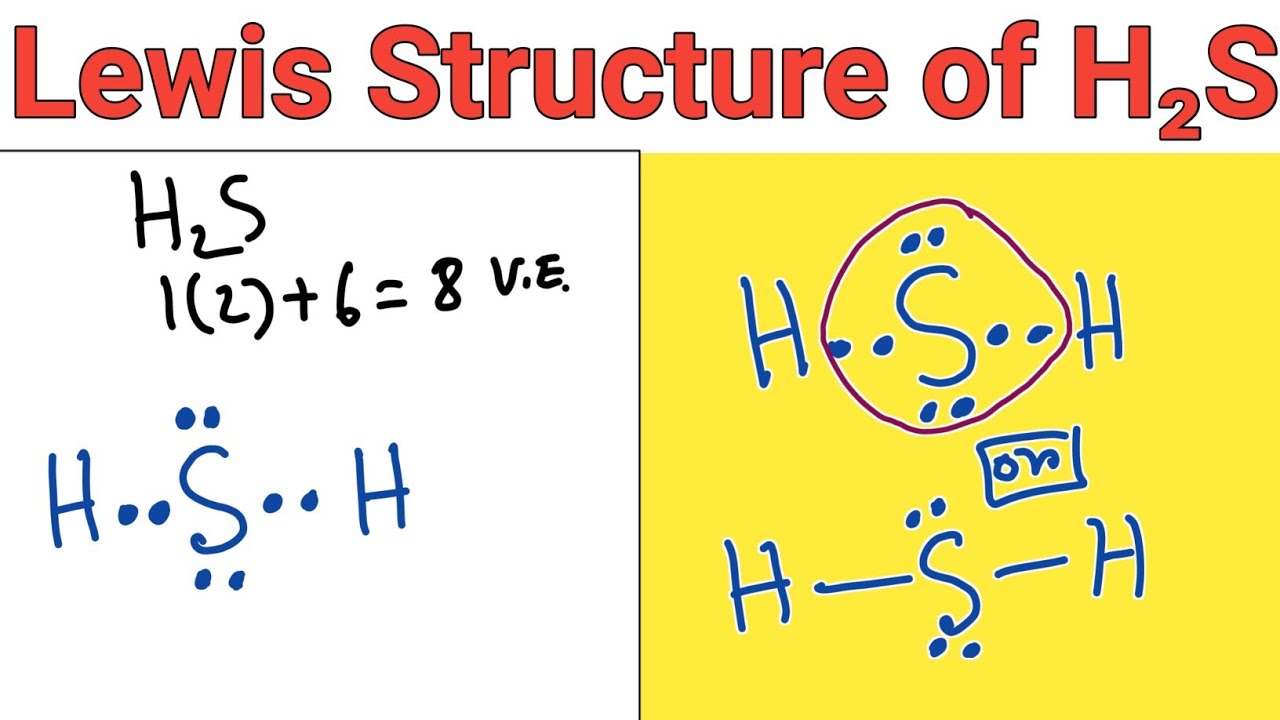
Lewis structure of H2S (Hydrogen sulphide) YouTube
Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
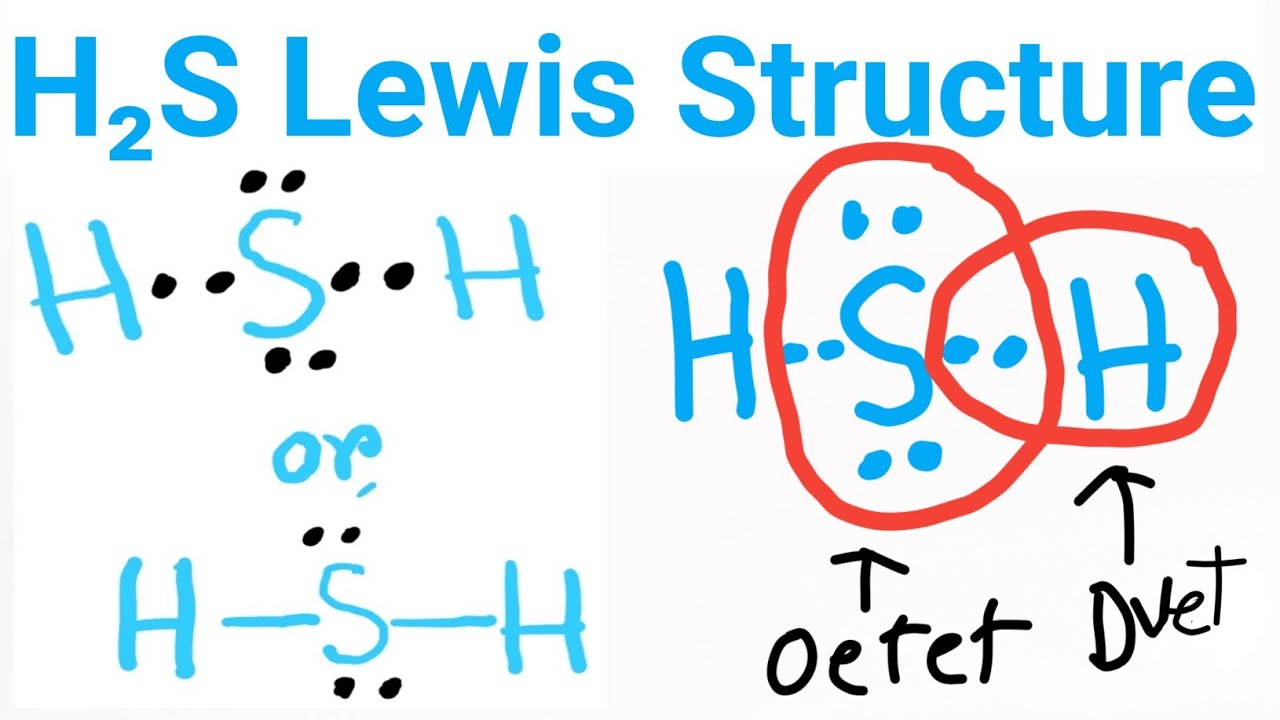
H2S Lewis Structure Lewis Dot Structure for H2S Hydrogen sulfide Lewis Structure YouTube
H2s lewis structure angle. In the H2S lewis structure, the intermixing 3s, 3p orbital form sp3 hybridized orbital, so the covalent bond angle should be 109.5̊ but it is lowered to 92.1̊ by the steric repulsion between dense two non bonding electron pair of 'S'. ad. For decreasing the bond angle (angle between the overlapping bonding.