
Products
HX2O +ClX2OX5 2HClOX3 H X 2 O + C l X 2 O X 5 2 H C l O X 3. The statement in the first article and the simple fact that there is such a dearth of information about this compound suggest that it really only exists as a transient, unstable, oxidizing acid that apparently has not been isolated as a stable compound.

WANIBESAK Antimon Pentaoksida Manfaat, Sifat Fisika, Pembuatan, Reaksi Kimia
Dichlorine Pentoxide - Cl 2 O 5. Cl2O5 Molar Mass Cl2O5 Oxidation Number. Reaction Expressions. Equilibrium Constant & Reaction Quotient. K c or Q = ( [Cl 2 O 5] 2) / ( [Cl 2] 2 [O 2] 5) (assuming all reactants and products are aqueous. substitutue 1 for any solids/liquids, and Psubstance for gases.)

SOLVEDWrite the formulas of the following compounds. a. nitrogen tribromide b. xenon tetroxide
Study with Quizlet and memorize flashcards containing terms like Which of the following statements correctly describe how to name a binary molecular compound? Select all that apply., The correct formula for the compound dichlorine pentoxide is Cl ___ O ___. Enter your answer in the correct format., Some covalent compounds are so common that they are named using "common" or "trivial" names.

Binary Molecular Compounds ppt download
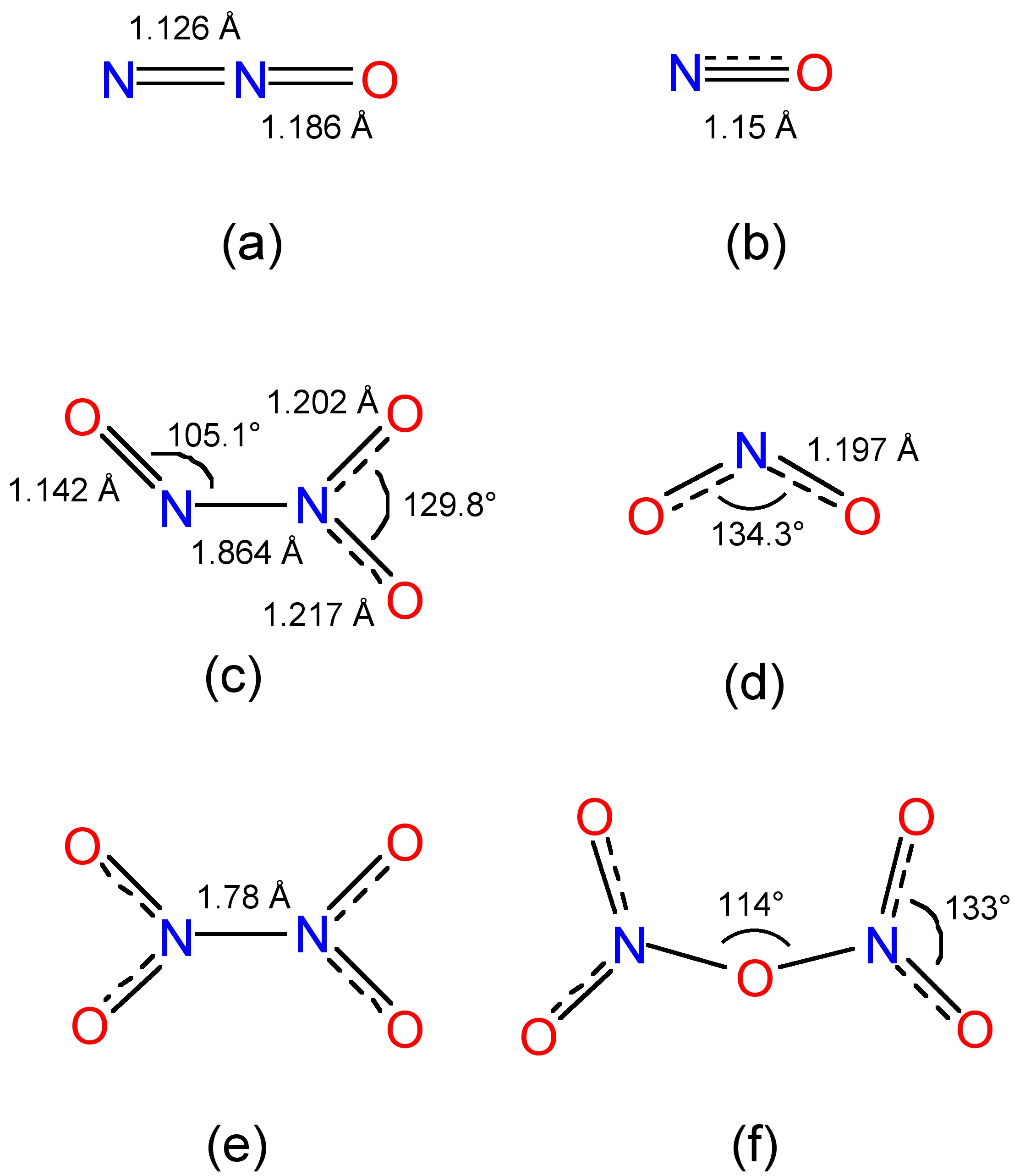
Dichlorine pentoxide is a hypothetical chlorine oxide with a chemical formula Cl 2 O 5. The most stable configuration of dichlorine pentoxide is unknown, but theory predicts that the perchloryl /chloride peroxide structure would be the most stable among various isomers, [1] such as the anhydride of chloric acid or the chlorous acid / perchloric.

How to Write the Formula for Dichlorine pentoxide YouTube
Dichlorine pentoxide is a highly reactive compound. It is a powerful oxidizing agent and can react violently with various substances, such as water, organic compounds, reducing agents, and even some metals. This compound is highly reactive and has the potential to be a powerful oxidizing agent. When it comes into contact with water, it reacts.
8.4 Oxides and Oxoacids Chemistry LibreTexts
Dichlorine pentoxide is a hypothetical chlorine oxide with a chemical formula Cl 2 O 5. The most stable configuration of dichlorine pentoxide is unknown, but theory predicts that the perchloryl /chloride peroxide structure would be the most stable among various isomers, [1] such as the anhydride of chloric acid or the chlorous acid / perchloric.
Chlorine Trifluoride Dichlorine Monoxide Chemistry Chloride, PNG, 1100x891px, Chlorine
Dichlorine heptoxide is the chemical compound with the formula Cl 2 O 7.This chlorine oxide is the anhydride of perchloric acid.It is produced by the careful distillation of perchloric acid in the presence of the dehydrating agent phosphorus pentoxide:. 2 HClO 4 + P 4 O 10 → Cl 2 O 7 + H 2 P 4 O 11. The chlorine(VII) oxide can be distilled off from the mixture.
Dichlorine Monoxide Arsenic Pentoxide Hypochlorous Acid, PNG, 1100x802px, Dichlorine Monoxide
Dichlorine pentoxide is a hypothetical chlorine oxide with a chemical formula Cl2O5. The most stable configuration of dichlorine pentoxide is unknown, but theory predicts that the perchloryl/chloride peroxide structure would be the most stable among various isomers, such as the anhydride of chloric acid or the chlorous acid/perchloric acid mixed anhydride.

Diklorin Pentaoksida Mempunyai Rumus Kimia UNDIGI
Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in Cl2O5: Molar Mass (g/mol) Cl (Chlorine) 2 × 35.453 = 70.906. O (Oxygen) 5 × 15.9994 = 79.997. 4. Sum Each Element's Mass. Finally, add together the total mass of each element to get the molar mass of Cl2O5:
PPT Nomenclature for Binary Compounds PowerPoint Presentation ID1155529
dichlorine trioxide, Cl2O3 as hypothetical isomer O−Cl−O−Cl−O, chlorine (III) oxide. dichlorine tetroxide, also known as chlorine perchlorate, Cl2O4 or ClOClO3, chlorine (I,VII) oxide. dichlorine pentoxide, Cl2O5 or ClOOClO3, is hypothetical. dichlorine hexoxide or chloryl perchlorate, Cl2O6 or [ClO2]+[ClO4]−, chlorine (V,VII) oxide.

PPT Chapter 4 Compounds and Their Bonds PowerPoint Presentation, free download ID1267929
Senyawa Diklorin Pentaoksida termasuk senyawa yang sangat berbahaya jika tidak ditangani dengan benar. Senyawa ini dapat menimbulkan iritasi pada kulit, mata, dan saluran pernapasan. Jika terhirup dalam jumlah banyak, senyawa ini dapat menyebabkan kerusakan paru-paru dan bahkan kematian. Oleh karena itu, penting untuk selalu menggunakan alat.

PPT Chap 8 PowerPoint Presentation, free download ID1277441
In this video we'll write the correct formula for Dichlorine pentoxide (Cl2O5).To write the formula for Dichlorine pentoxide we'll use the Periodic Table and.
Dichlorine Monoxide Arsenic Pentoxide Hypochlorous Acid PNG, Clipart, Anhidruro, Arsenic
Dichlorine Pentoxide Cl2O5 Molar Mass, Molecular Weight. Cl2O5 Molar Mass Converter

30+ Hydrochloric Acid And Sodium Hydroxide Equation Insende
Chlorine forms a series of oxides (Table 10.3. 2) in which the chlorine has the formal oxidation states +1, +4, +6, and +7. The physical properties of the oxides are summarized in Table 10.3. 2. While, the oxides of chlorine are not very stable (in fact several are shock sensitive and are prone to explode) the conjugate oxyacids are stable.

PPT Mixed Formula Practice PowerPoint Presentation, free download ID2170520
= Dichlorine pentoxide (Cl 2 O 5) Perchloric acid (HClO 4) = Dichlorine heptoxide (Cl 2 O 7) Dichlorine monoxide, Cl 2 O, is one of the "chlorine-oxide" creators of the oxy-chlorine acids. It is a brownish yellow gas at room temperature that can explode at high concentrations when heated or subjected to sparks.

Rumus Kimia Sulfur Tetrafluorida Rumus Kimia
Dichlorine pentoxide (Cl. 2. O. 5. ) Molecule Lewis Structure. Dichlorine pentoxide (Cl 2 O 5) contains two chlorine and five oxygen atoms. In the lewis structure of Cl 2 O 5 molecule there are three Cl=O bonds. One chlorine atom is located as a center atom and other chlorine atom is located in a side of the molecule.