PPT Periodic Classification of Elements PowerPoint Presentation, free download ID1876801
furfural. magnesium. triad. Johann Wolfgang Döbereiner (born Dec. 13, 1780, Hof an der Saale [Germany]—died March 24, 1849, Jena) was a German chemist whose observation of similarities among certain elements anticipated the development of the periodic system of elements. As a coachman's son, Döbereiner had little opportunity for formal.

Dasar Hukum Triade Hukum 101
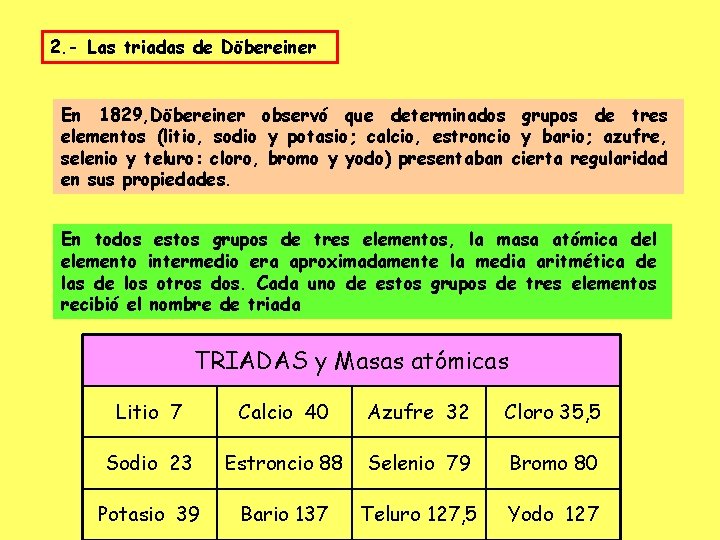
Contoh hukum triade dobereiner yang lain ini adalah pada golongan unsur alkali tanah. Unsur-unsur tersebut adalah berlium (Be), magnesium (Mg), dan kalsium (Ca). Ketiga unsur ini dijadikan ke dalam satu kelompok karena memiliki persamaan sifat dan digolongkan ke dalam unsur alkali tanah. Perhitungan ketiga unsur ini dan tabel teori dibereiner.

(DOC) Triade Dobereiner Salma Aqilla Academia.edu
Ans. The correct answer is A. Arsenic Explanation: According to the Dobereiner triad, the atomic mass of the third element can be found using the arithmetic mean of the two elements i.e. phosphorous and antimony. We have, the atomic mass of phosphorous is 31 and the atomic mass of antimony is 121.75. Therefore the atomic mass of the third element = (31+121.75)/2 =76.37
PPT Historical Development of the Periodic Table PowerPoint Presentation ID1481918
Hence they form Dobereiner's triad. Question 6: State the alkaline earth metal Dobereiner's triad. Answer: The alkaline earth metals that are a part of the Dobereiner triads are calcium, strontium and barium. Question 7: Verify the law of triads for the alkali metals triad. Answer: The alkali metals triad consists of Lithium. Sodium and.

Trick to learn Doberenier Triad / dobereiner's law of triads / NEET chemistry video lecturer
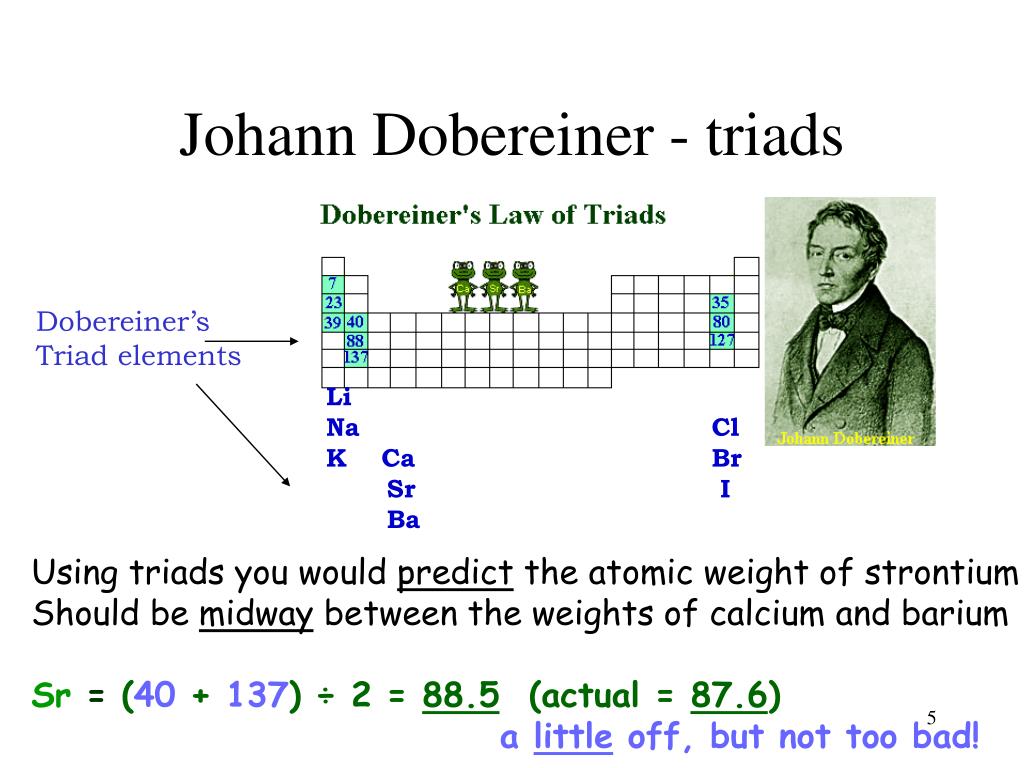
In the history of the periodic table, Dobereiner's triads were an early attempt to sort the elements into some logical order by their physical properties. In 1817, a letter reported Johann Wolfgang Dobereiner's observations of the alkaline earths; namely, that strontium had properties that were intermediate to those of calcium and barium. By 1829, Dobereiner had found other groups of three.
Que Son Las Triadas De Dobereiner bourque
Menurut Triade Dobereiner massa atom unsur yang ditengah (Li) merupakan rata-rata massa atom unsur lithium dan kalium. Perhatikan contoh perhitungan berikut: Unsur. Massa Atom. Rata Rata Massa Atom Unsur 1 dan 3. Unsur Pertama. Lithium (Li) 6,94. = (6,94+39,10) : 2 = 23,02.

Dobereiner's Triads Newland Law of Octaves Periodic Classification of Elements ClassX
Berisi penjelasan tentang teori triade Dobereiner. Dapatkan materi dan soal yang lebih lengkap di www.aguskamaludin.com

Dobereiner's Triads 02 YouTube
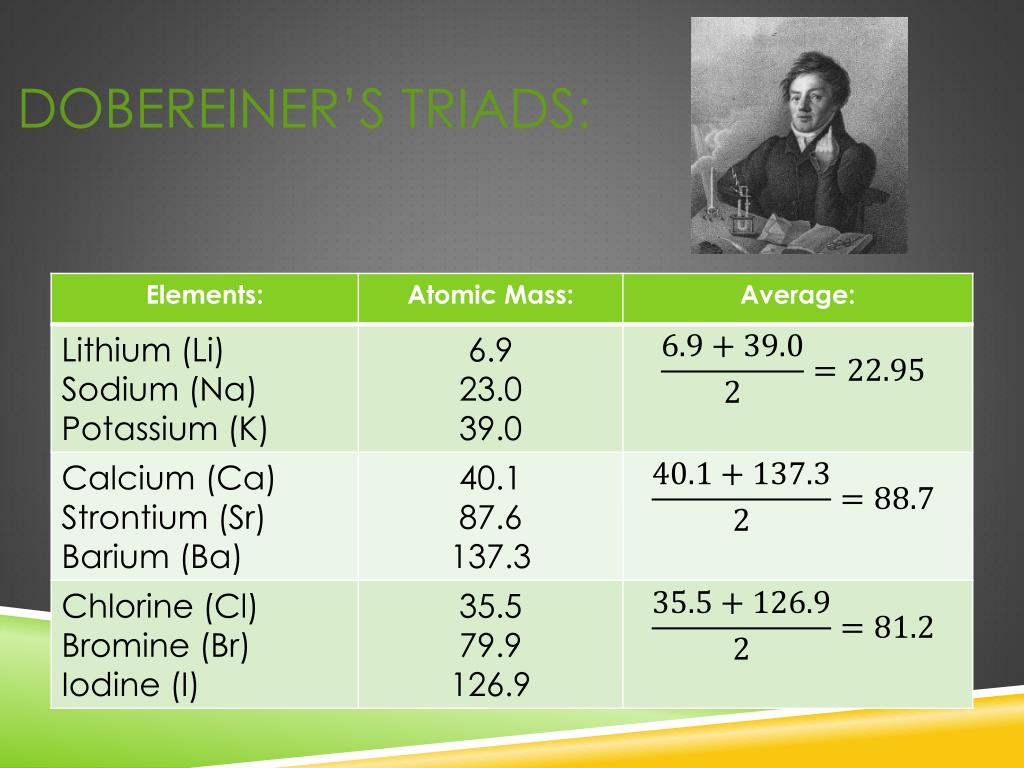
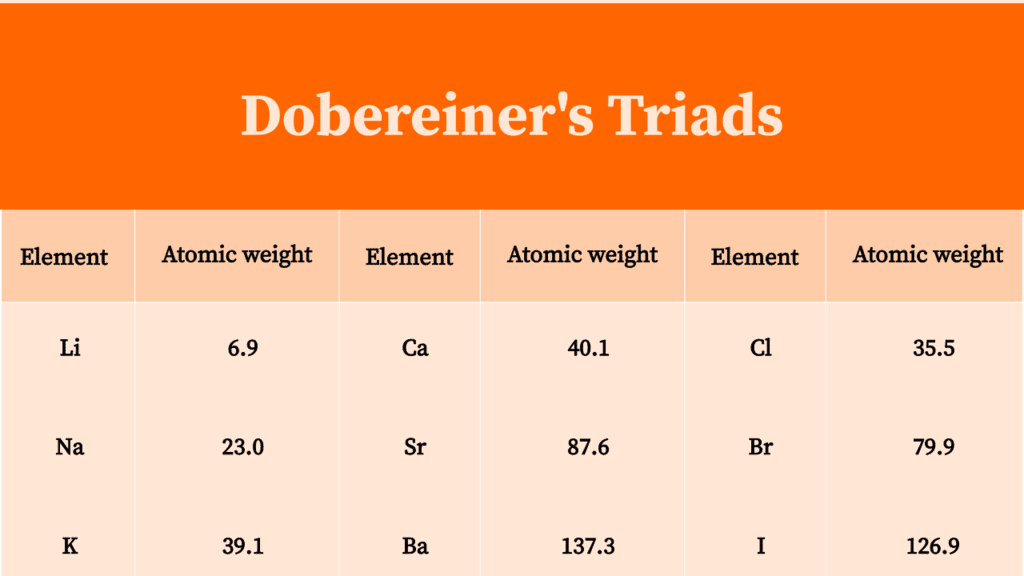
Dobereiner's law of triads states that, the atomic mass of the middle element of a triad is the arithmetic mean of the atomic masses of the other two elements. Example: In the triad of lithium, sodium and potassium. The atomic mass of lithium is 7 and the atomic mass of potassium is 39. The average of masses of lithium and potassium gives.

Periodic Classification of Elements 2 Dobereiner's Triads CBSE Class 10 YouTube
Contoh Triade Dalam golongan logam alkali, pada awalnya dobereiner mengelompokkan unsur lithium (li), natriu (Na), dan kalium m (K) menjadi satu kelompok. Ketiganya memiliki kemiripan sifat, berupa unsur logam, reaktif, bervalensi +1, dan sebagainya. Menurut Triade Dobereiner massa atom unsur yang ditengah (Li) merupakan rata-rata massa atom.

Dasar Pengelompokan Unsur Triade Dobereiner dan sistem Oktaf Newlands YouTube
Triade Dobereiner (Artikel Lengkap) Follow @HediSasrawan. Dalam sejarah tabel periodik, triade dobereiner adalah upaya paling pertama dalam hal pengelompokan unsur-unsur kimia dengan sifat fisik tertentu. Pada tahun 1829, kimiawan asal Jerman Johann Wolfgang Döbereiner mencatat bahwa ada triad unsur yang menunjukkan sifat kimia yang mirip.

Pengelompokan Unsur Triade Dobereiner dan Oktaf Newlands 0 TABEL PERIODIK Tujuan Pembelajaran
By 1829, Döbereiner had found other groups of three elements (hence "triads") whose physical properties were similarly related. He also noted that some quantifiable properties of elements (e.g. atomic weight and density) in a triad followed a trend whereby the value of the middle element in the triad would be exactly or nearly predicted by taking the arithmetic mean of values for that.
Dobereiner's Triads, Octaves and Mendeleev's Periodic Table Class 10 Notes EduRev
Hukum Triad Dobereiner adalah sebuah hukum atau teori yang mengelompokkan tiga unsur kimia dengan kemiripan sifat tertentu. Pada tahun 1829, Johann Wolfgang Dobereiner, melihat adanya kemiripan sifat dan massa atom antara beberapa unsur. Ia kemudian membentuk kelompok yang terdiri dari tiga unsur dengan sifat kimia yang mirip.

Döbereiner's Triads School Of Elements Part 2 YouTube
Kelemahan dari Triade Dobereiner adalah sifat unsur yang dia pelajari hanya berfokus pada kelompok kesamaan sifat unsur yang beranggotakan tiga unsur. Dia tidak dapat menjelaskan hubungan antara triade yang satu dengan triade yang lainnya. Aturan dobereiner didasarkan pada. Setiap group terdiri dari tiga unsur yang memiliki sifat fisika sama
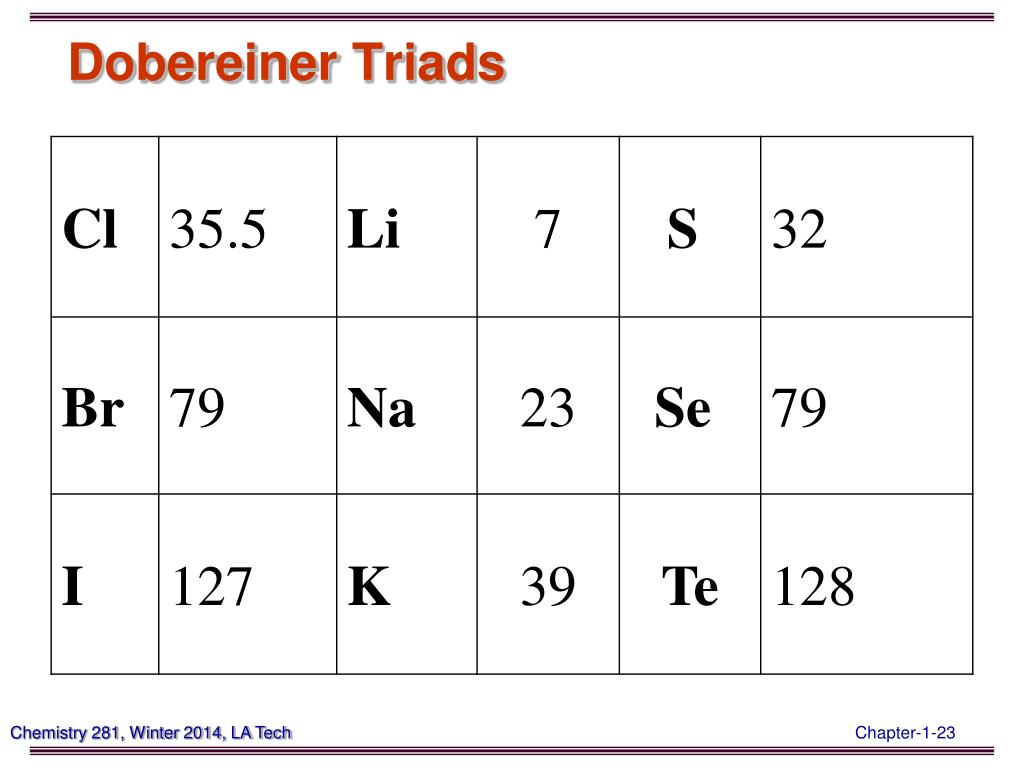
PPT Chemistry 281(01) Winter 2014 PowerPoint Presentation, free download ID4995358
This was known as the Dobereiner's law of triads. The law states that when elements are placed in the ascending order of atomic masses, groups of three elements having similar properties are obtained. The atomic mass of the middle element of the triad is equal to the mean of the atomic masses of the other two elements of the triad.
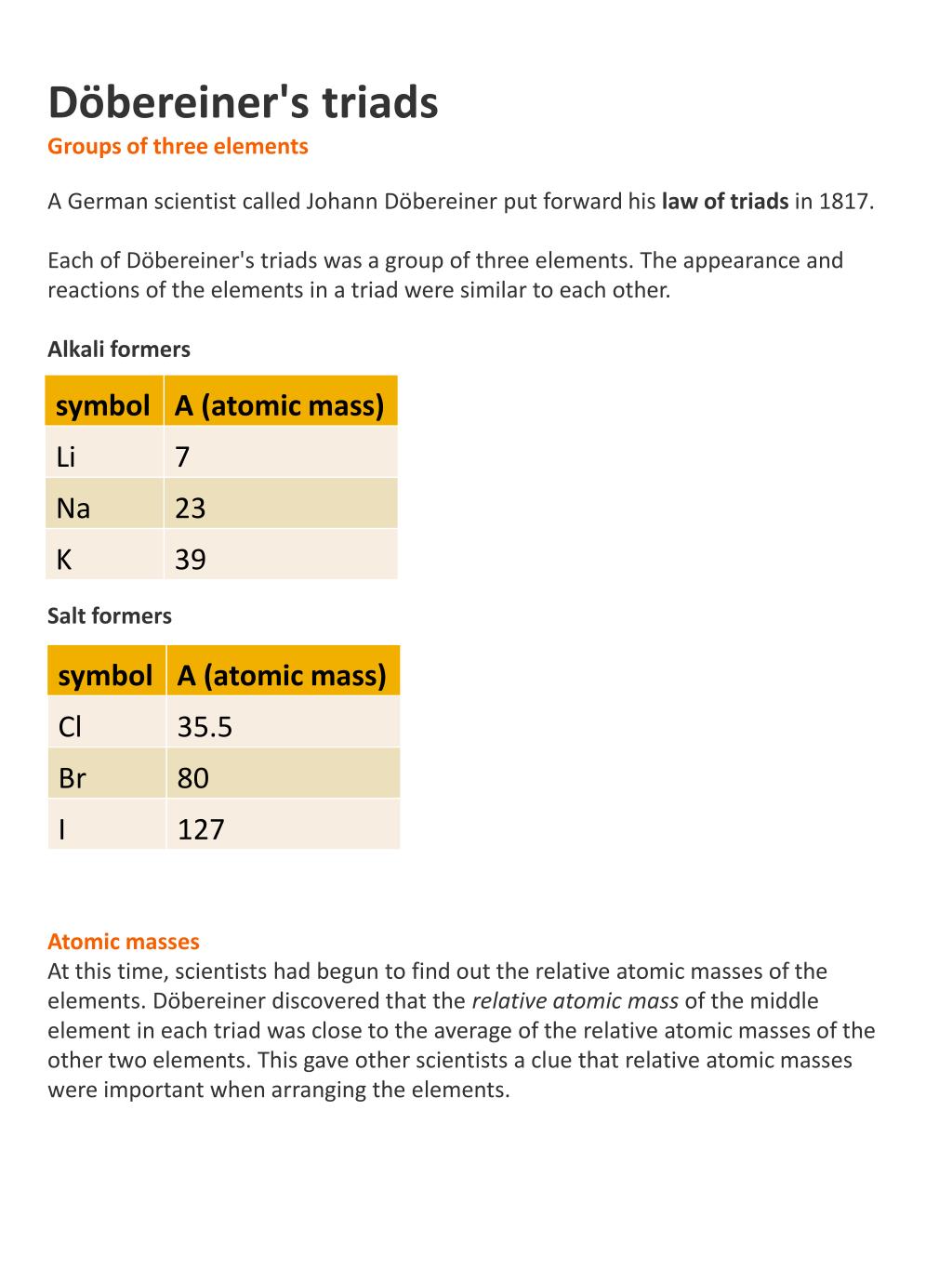
PPT Döbereiner's triads Groups of three elements PowerPoint Presentation ID2856964
Pengelompokan Unsur Triade Dobereiner. Pada tahun 1817, Johann Wolfgang Dobereiner mengelompokkan unsur-unsur berdasarkan kenaikan massa dan kesamaan sifatnya.. Contoh: Jari-jari atom 19 K lebih besar daripada jari-jari atom 3 Li. Kon gurasi atom kalium adalah 2 8 8 1 dengan 4 kulit, sedangkan kon gurasi atom litium adalah 2 1 dengan 2 kulit.
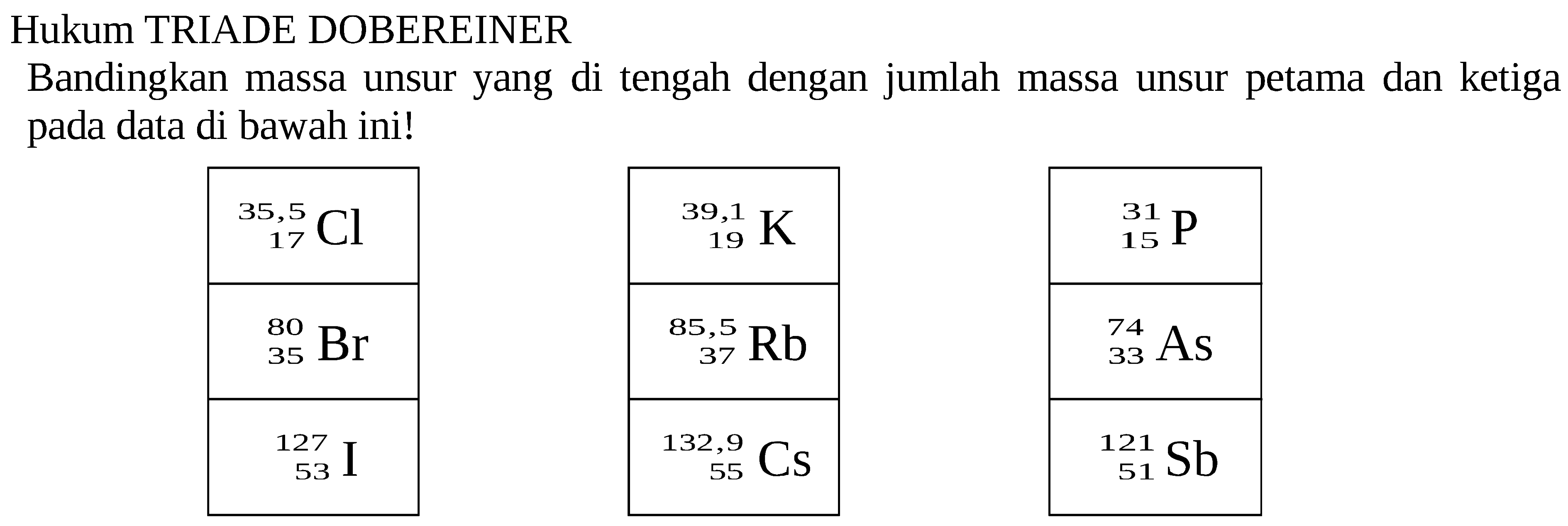
Hukum TRIADE DOBEREINER Bandingkan massa unsur yang di te...
Quick Reference. A set of triads of chemically similar elements noted by Johann Döbereiner (1780-1849) in 1817. Even with the inaccurate atomic mass data of the day it was observed that when each triad was arranged in order of increasing atomic mass, then the mass of the central member was approximately the average of the values for the.