
Estructura de Lewis NO2, Ejercicios Resueltos » Quimica Online
NO 2 molecule: The Lewis structure of NO 2 molecule is shown below. Figure 1.2h NO2 molecule Lewis structure. For above molecules, they all contain unpaired (single) electrons. The neutral species that contain an unpaired electron is called radical (or free radical). When the carbon atom of a alkyl group has an unpaired electron, the species is.
NO2 Lewis Structure Nitrite Ion YouTube
1.1K 208K views 6 years ago A step-by-step explanation of how to draw the NO+ Lewis Dot Structure (Nitronium ion). For the NO+ structure use the periodic table to find the total number of.
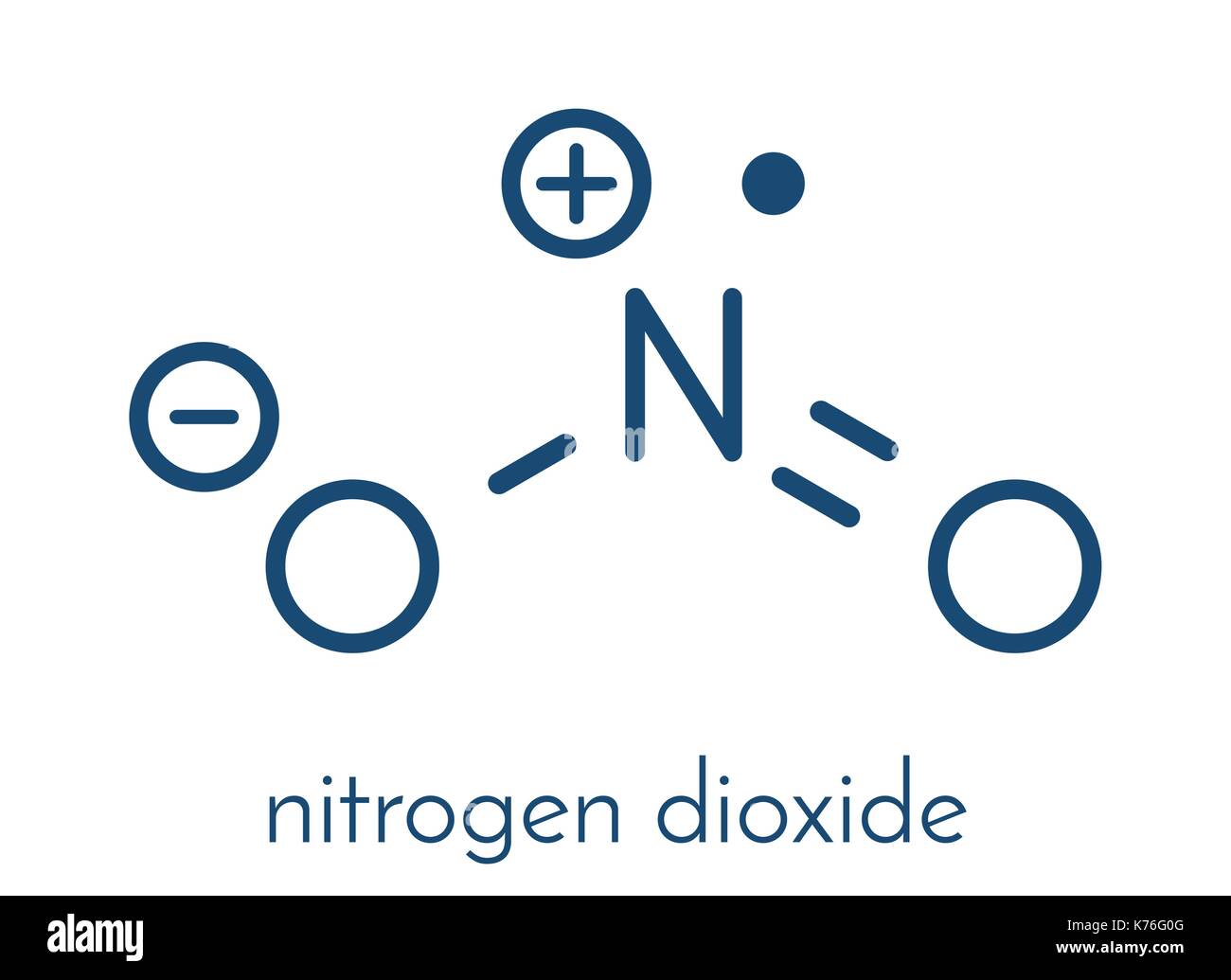
Chemical structure nitrogen dioxide no2 Stock Vector Images Alamy
The Lewis structure of NO2, which consists of one nitrogen atom bonded to two oxygen atoms with a double bond, influences its chemical properties. This structure implies that NO2 has a linear geometry and a formal charge of +1 on the nitrogen atom and -1 on each oxygen atom.

How to draw NO2+ Lewis Structure? Science Education and Tutorials
Let us talk about drawing the Lewis Structure for Nitrogen Dioxide ( NO2 ). Lewis Structure of NO2 A molecule of nitrogen dioxide consists of one nitrogen atom and two atoms of oxygen. Let us look at the periodic table. Nitrogen belongs to group 15 ( or group 5) and has an atomic number of 7, therefore has a valency of 5.
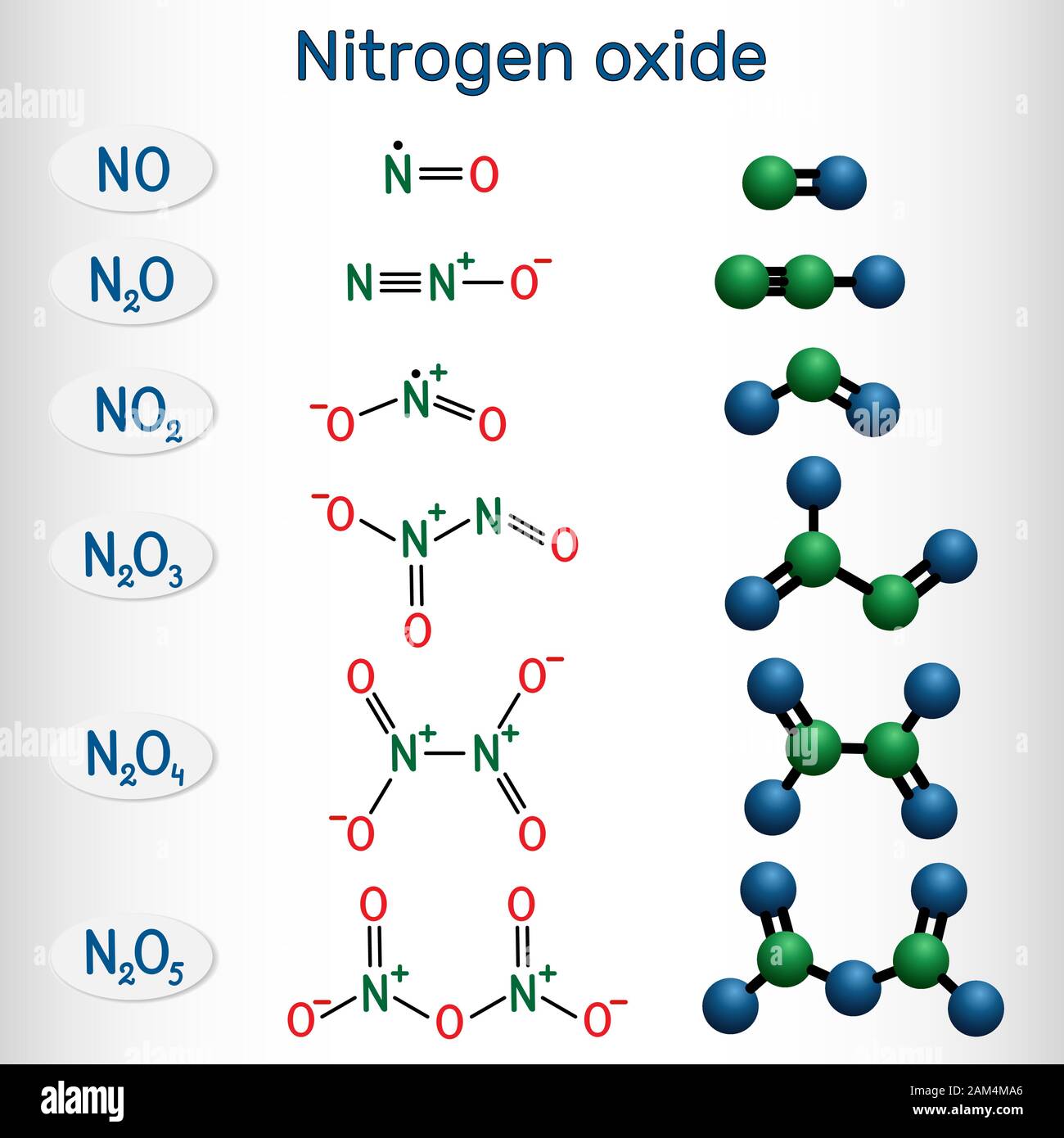
Chemical formulas and molecule model of nitrogen oxide nitric oxide NO
NO2 - Molecular Geometry / Shape and Bond Angles Wayne Breslyn 727K subscribers Join Subscribe Subscribed 556 Share 149K views 4 years ago A quick explanation of the molecular geometry of NO2 -.

NO2 Molecular Geometry / Shape and Bond Angles YouTube
This chemistry video tutorial explains how to draw the lewis structure of NO2-, the Nitrite ion.Chemistry - Basic Introduction: https://ww.
No2 Lewis Structure
The Lewis structure of a NO2- consists of a nitrogen (N) atom and two oxygen (O) atoms. The nitrogen (N) atom is present at the center of the molecular ion, while the two O-atoms occupy terminal positions. Meanwhile, there is a lone pair of electrons on the central N-atom.

Diagrama de Lewis del dioxido de nitrógeno NO2 YouTube
Key Takeaways The NO2 Lewis structure consists of a nitrogen atom bonded to two oxygen atoms. The nitrogen atom has a l one pair of electrons, while the oxygen atoms have three lone pairs each. The nitrogen-oxygen bonds are represented by single bonds, and the nitrogen-oxygen double bond is represented by a double bond.
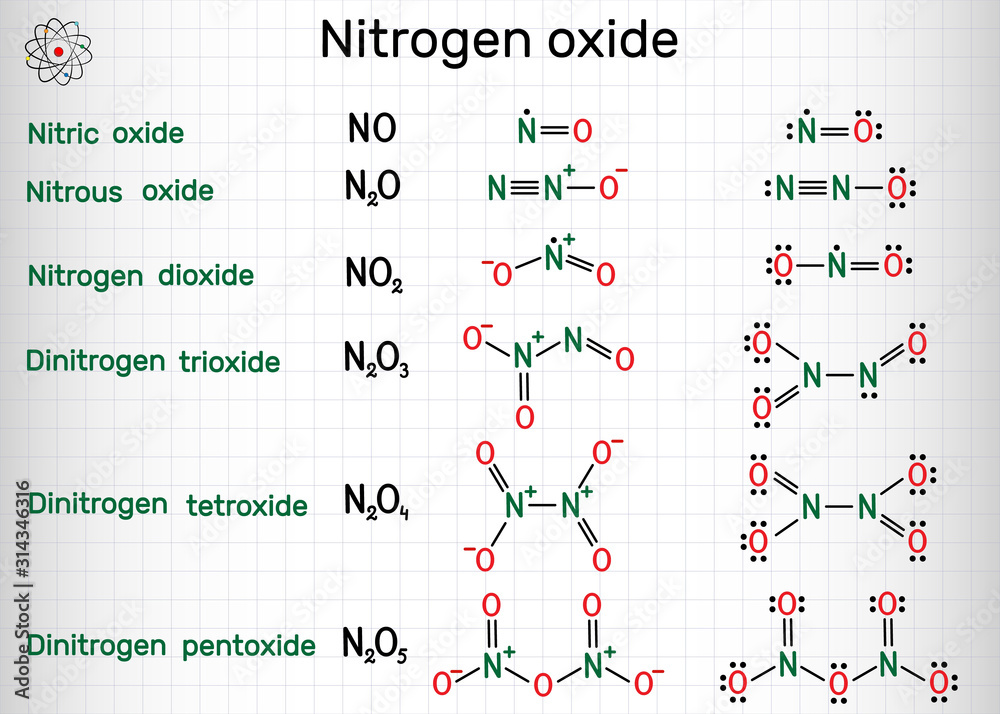
Chemical formulas of nitrogen oxide nitric oxide NO, nitrogen dioxide
Subscribed 2.4K 478K views 10 years ago A step-by-step explanation of how to draw the NO2 Lewis Structure (Nitrogen Dioxide). The NO2 Lewis structure has a total of 17 valence electrons..

Nitrogen dioxide, NO2, molecule model and chemical formula ⬇ Vector
A step-by-step explanation of how to draw the NO2 - Lewis Dot Structure (Nitrite ion). For the NO2 - structure use the periodic table to find the total number of valence electrons f.
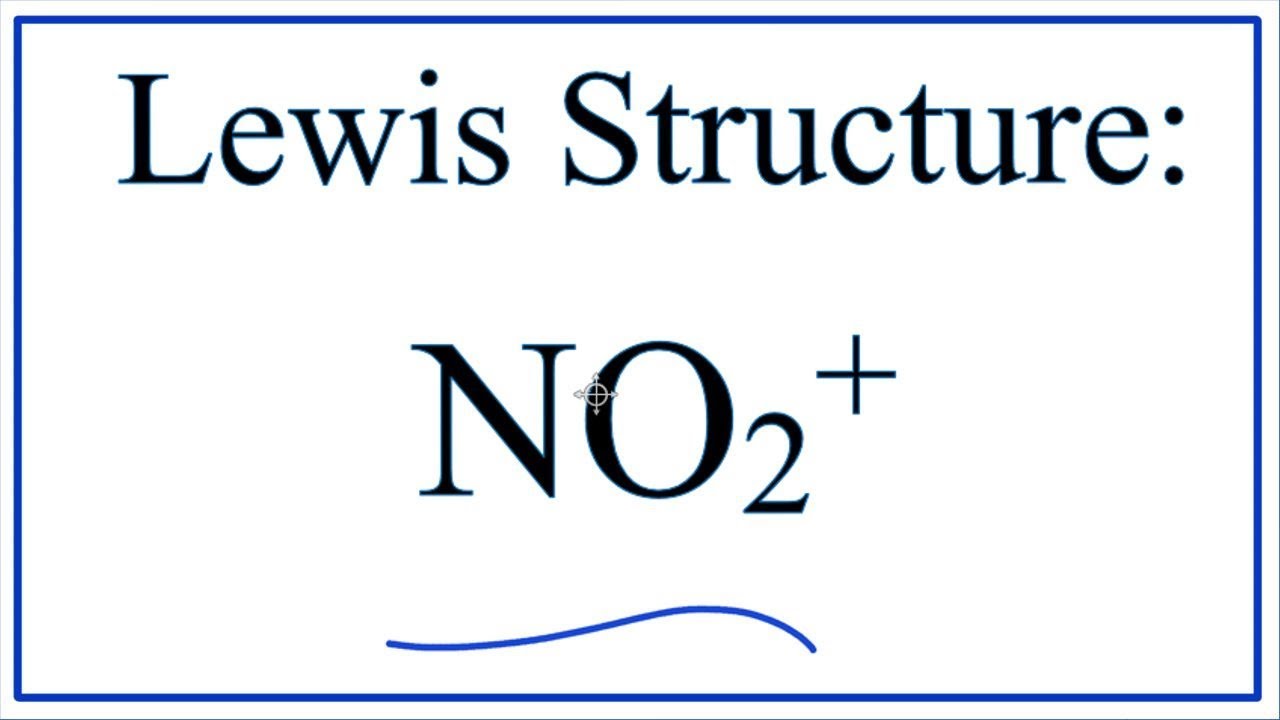
How To Draw The Lewis Structure of NO2+ (Nitronium Ion) YouTube
Welcome to Warren Institute! In this article, we will dive into the fascinating world of Chemistry and explore the NO2- Lewis Structure. As aspiring

No2 nitrogen dioxide molecule Royalty Free Vector Image
NO2- lewis structure has a Nitrogen atom (N) at the center which is surrounded by two Oxygen atoms (O). There is 1 double bond and 1 single bond between the Nitrogen atom (N) and each Oxygen atom (O). There is a -1 formal charge on the single bonded Oxygen atom (O).

Lewis Structure of NO2(1), the nitrite ion. YouTube
Nitrogen dioxide is a chemical compound with the formula NO2. One of several nitrogen oxides, nitrogen dioxide is a reddish-brown gas. It is a paramagnetic, bent molecule with C 2v point group symmetry.

No2 Lewis Structure
In the Lewis structure for NO2 the Nitrogen atom is the least electronegative atom and goes at the center of the structure. For the NO2 Lewis structure, calculate the total number of valence electrons for the NO2 molecule. After determining how many valence electrons there are in NO2, place them around the central atom to complete the octets.
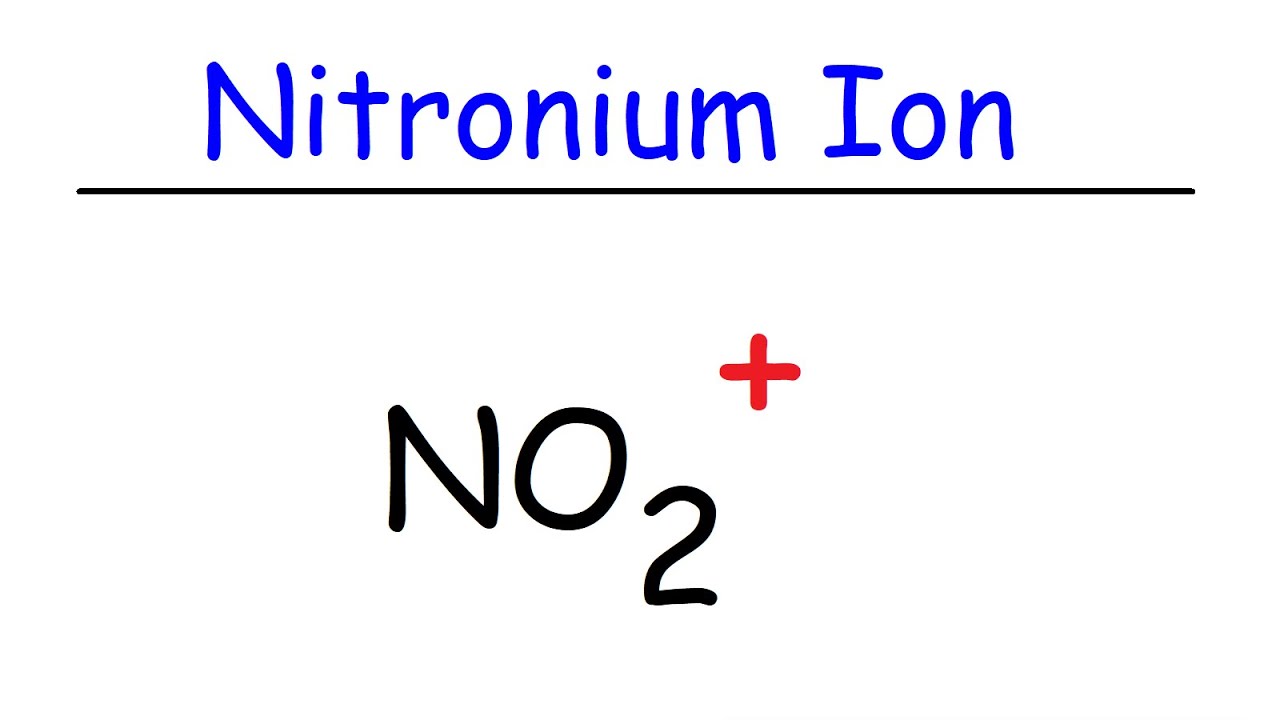
NO2+ Lewis Structure Nitronium Ion YouTube
NO 2 structure. The bond length between the nitrogen atom and the oxygen atom is 119.7 pm. That means both bonds exist between oxygen and nitrogen are same. Due to exist of unpaired electron, NO 2 is a free radical. There is a unpaired electron on nitrogen atom in NO2 lewis structure.
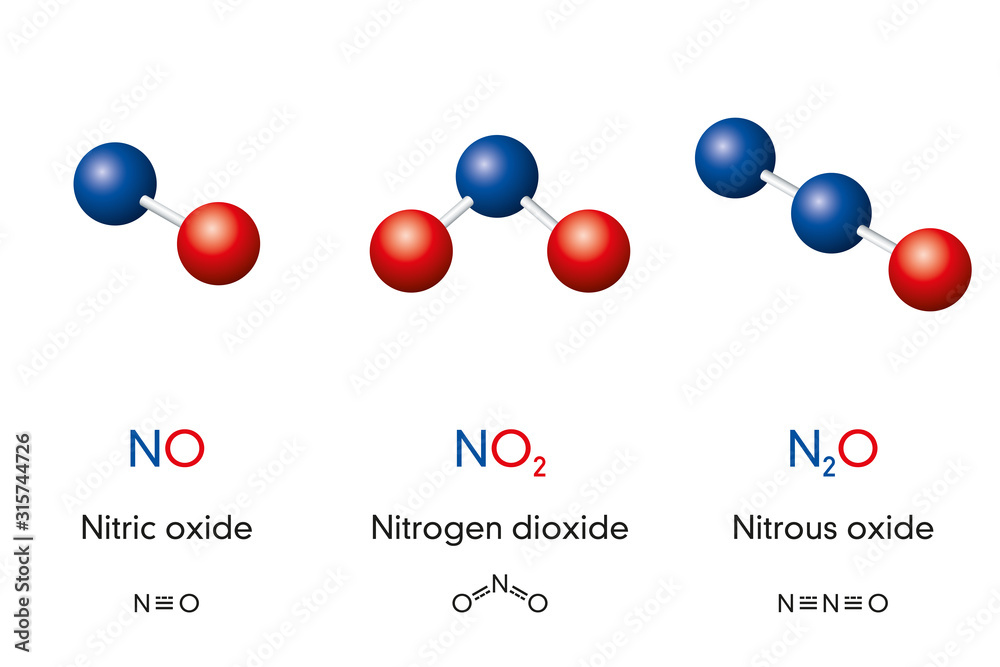
Fototapeta Nitric oxide NO, Nitrogen dioxide NO2 and Nitrous oxide N2O
Lewis dot structure: A Lewis Structure, also known as an Electron Dot Structure, is a simplified representation of a molecule's valence shell electrons. It describes the arrangement of valence electrons around particular atoms in a molecule. Electronic configuration of nitrogen = 1 s 2 2 s 2 2 p 3 and electronic configuration of oxygen = 1 s 2.