
Alkil Adalah Ujian
Pengalkilan atau alkilasi ialah tindak balas kimia yang melibatkan pemindahan kumpulan alkil. Kumpulan alkil boleh dipindahkan sebagai alkil karbokation, radikal bebas, karbanion atau karbena (atau setara dengannya). [1] Agen pengalkilan ialah reagen untuk melaksanakan pengalkilan. Kumpulan alkil juga boleh disingkirkan dalam proses yang.
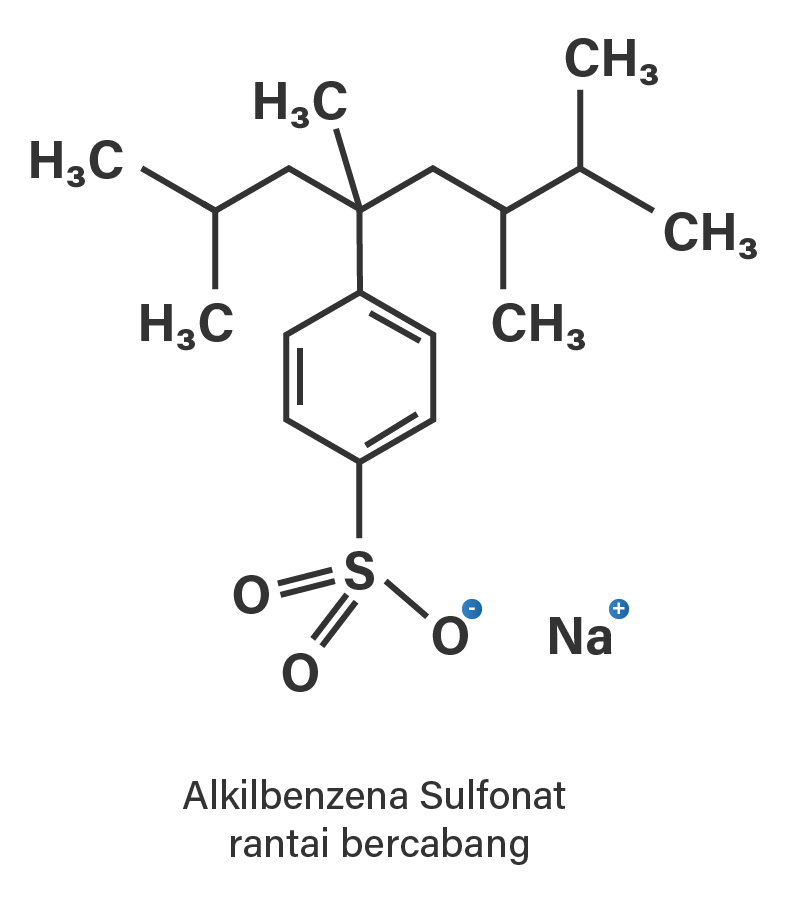
gambar struktur dari alkil benzena sulfonat...
Alkyl group is formed by removing a hydrogen atom from the molecule of alkane. Alkanes are quite often represented as R-H and here R stands for alkyl group. The general formula of the alkyl group is CnH2n+1. The smallest alkyl group is CH 3 called methyl. A cycloalkyl is derived from a cycloalkane by removal of a hydrogen atom from a ring.
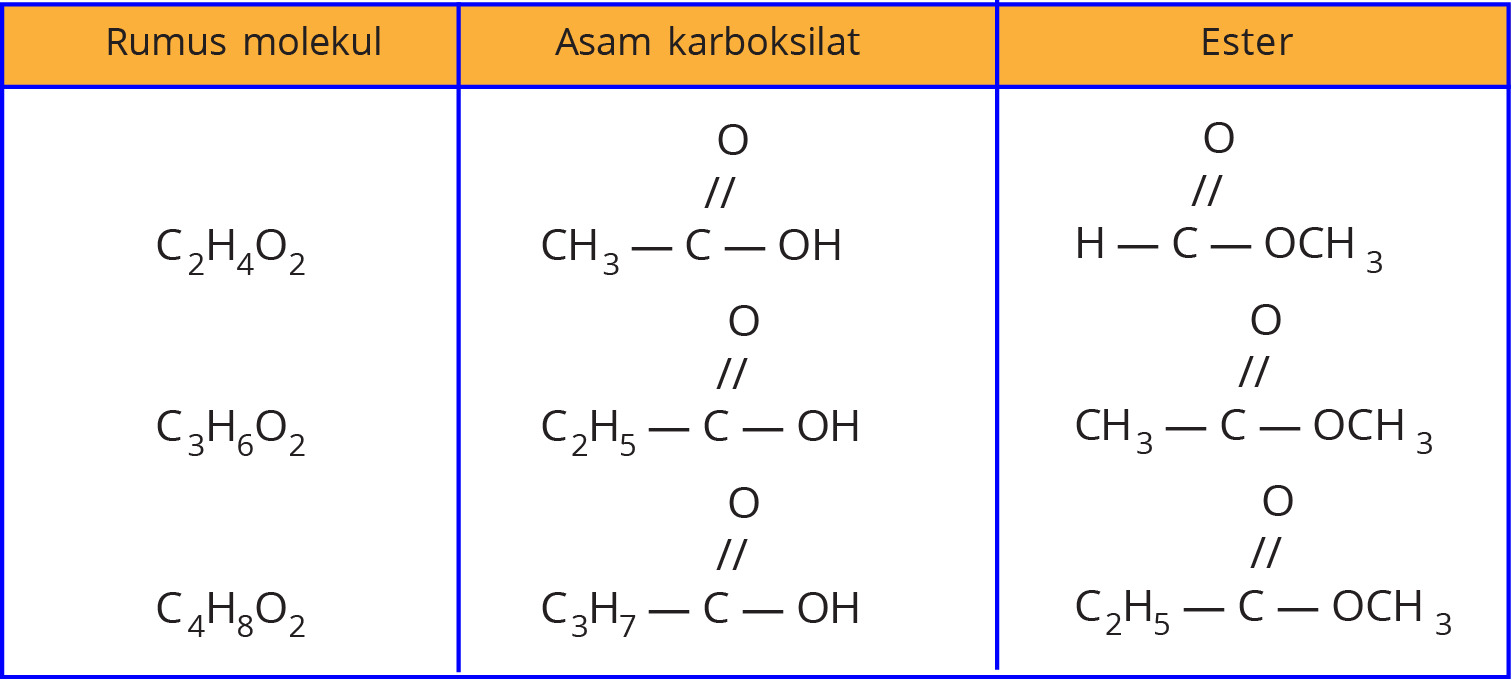
Isomer Asam Alkanoat dan Alkil Alkanoat Materi Kimia
We work with the main car rental companies and with local rental companies to be able to offer you the widest variety of cars and prices. Thus, we guarantee you the best car rental offers in more than 150 countries around the world.

Alkyl Halide Reaction Map And Summary Organic chemistry study, Organic chemistry, Chemistry
Sadržaj. Alkil. Alkilni supstituent - u organskoj hemiji - je alkan kojem nedostaje jedan atom vodika. Termin alkil je namjerno nespecifično uključen u mnoge moguće supstituente. [1] Aciklični alkil ima opću formulu C n H 2n+1. Cikloalkil je izveden iz cikloalkana, uklanjanjem vodikovog atoma iz prstena i ima opću formulu C n H 2n−.
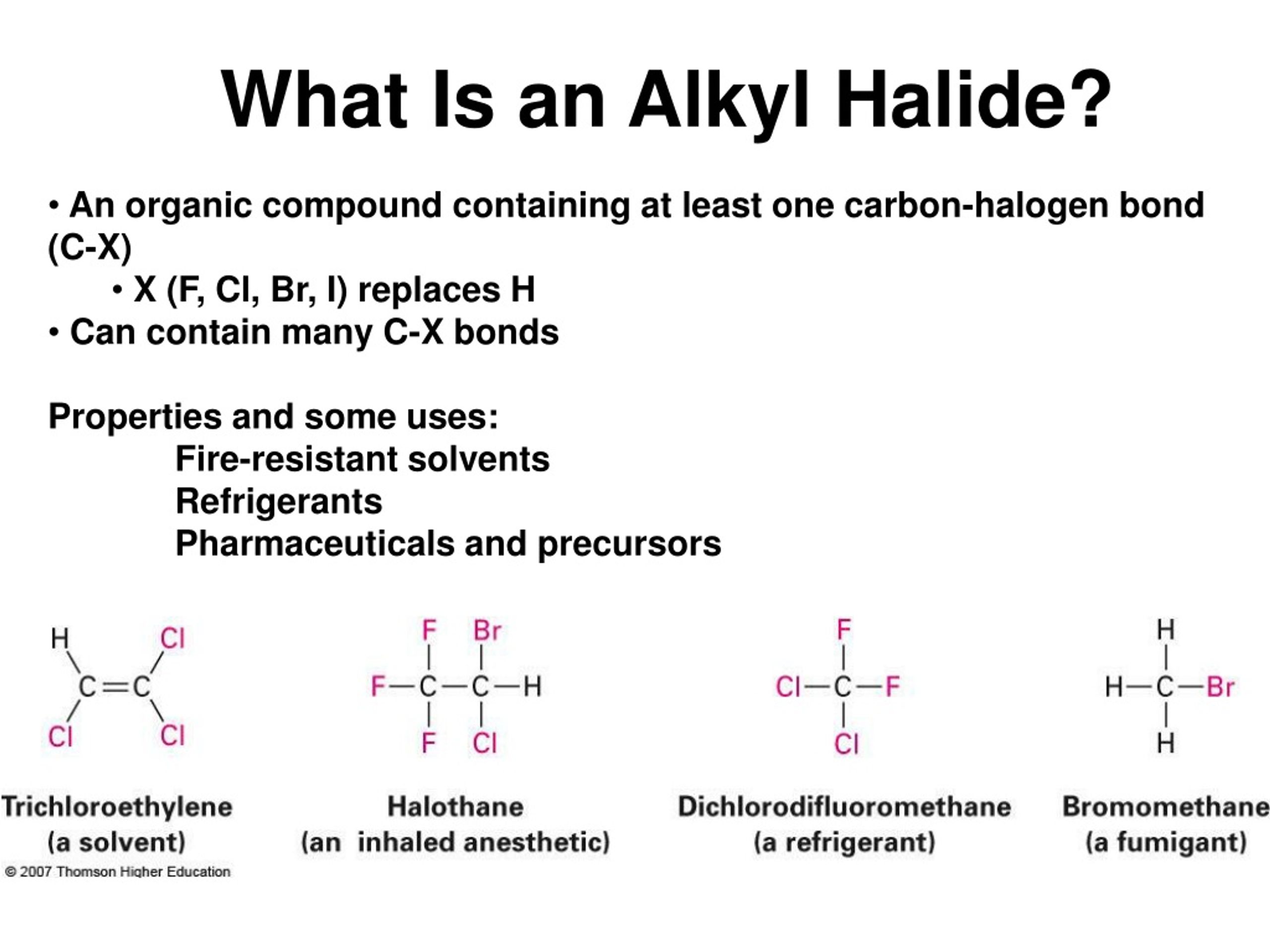
PPT Alkyl Halides PowerPoint Presentation, free download ID153595
Examples of some common alkyl groups are given in the following table. Note that the "ane" suffix is replaced by " yl " in naming groups. The symbol R is used to designate a generic (unspecified) alkyl group. Table 3.3.1 3.3. 1: Alkyl Group names. Group. CH 3 -. C 2 H 5 -. CH 3 CH 2 CH 2 -. (CH 3) 2 CH-.

PPT Alkyl Halides PowerPoint Presentation, free download ID2027688
As mentioned earlier, an alkyl group is an alkane missing one of its hydrogen atoms. They are formed through the removal of a hydrogen atom from the alkane structure. The general formula of.
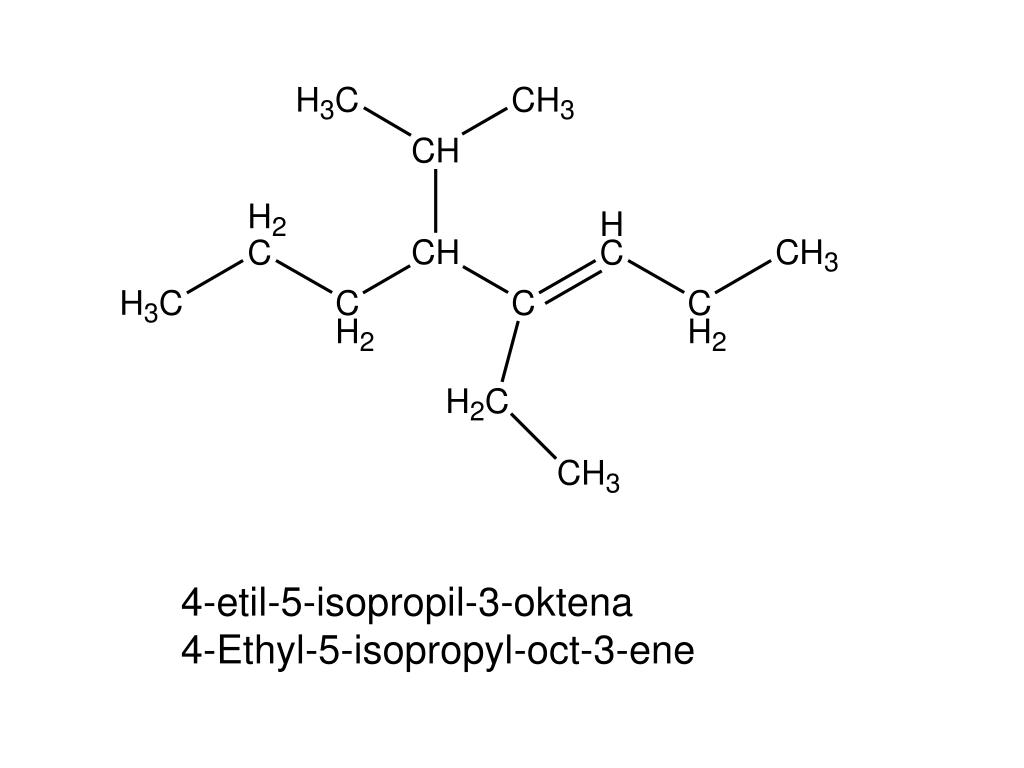
PPT GUGUS ALKIL sebagai cabang dengan rumus C n H 2n+1 PowerPoint Presentation ID5459599
Alkil. Izopropil grupa. Metil grupa. U organskoj hemiji, alkil supstituent je alkan bez atoma vodonika. [1] Termin alkil je namerno nespecifičan da bi obuhvatio mnoge moguće supstituente. Aciklični alkil ima opštu formulu C n H 2n+1. Cikloalkil je izveden iz cikloalkana uklanjanjem atoma vodonika sa prstena i ima opštu formulu C n H 2n-1. [2]

Mengenal Alkil dan Aril Halida
Alkanlardan bir hidrojen kopması ile oluşan gruplara Alkil denir.. Açık zincirli genel formülleri CnH2n+1'dir.. Radikal gruplardır. R ile gösterilir.. Karasızdırlar. Alkillerin adlandırılmasında ön ekin sonuna, alkanlardaki an eki yerine il eki getirilir.. IUPAC'a göre ilk 4 alkan özel olarak isimlendirilmiştir.
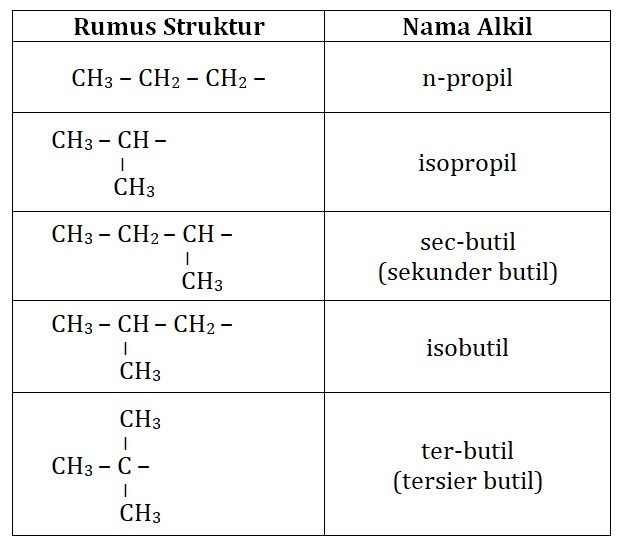
Gugus Alkil ( R)
Gugus Alkil. Gugus alkil adalah alkana yang telah kehilangan satu atom H. Gugus alkil ini dapat dituliskan dengan menggunakan rumus: CnH2n + 1. Dengan menggantikan satu atom H, maka namanya juga akan berubah dari metana menjadi metil. Berikut ini beberapa gugus alkil yang biasa digunakan. Rumus. Nama Alkil. CH3 -.

PPT ALKIL HALIDA Tinjauan reaksi subtitusi nukleofilik PowerPoint Presentation ID3820295
Alkylphenol. Alkylphenols are a family of organic compounds obtained by the alkylation of phenols. The term is usually reserved for commercially important propylphenol, butylphenol, amylphenol, heptylphenol, octylphenol, nonylphenol, dodecylphenol and related "long chain alkylphenols" (LCAPs). Methylphenols and ethylphenols are also.

Nomenclature and classification of alkyl halides YouTube
Alkyl group. In organic chemistry, an alkyl group is an alkane missing one hydrogen. [1] The term alkyl is intentionally unspecific to include many possible substitutions. An acyclic alkyl has the general formula of −CnH2n+1. A cycloalkyl group is derived from a cycloalkane by removal of a hydrogen atom from a ring and has the general formula.

Image result for alkyl group Chemistry, Organic chemistry, Molecular
The LibreTexts libraries are Powered by NICE CXone Expert and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739.

Mengenal Alkil dan Aril Halida
Alkyl halides are halogen-substituted alkanes wherein one or more hydrogen atoms of an alkane is replaced by a halogen atom such as fluorine, chlorine, bromine, or iodine. The carbon atom in an alkyl halide is bonded to the halogen atom, which is sp3 -hybridized and exhibits a tetrahedral shape. Unlike alkyl halides, compounds in which a.

39 Tabel Rumus Alkil Dan Nama Alkil paling Lengkap
Tabel Perbedaan Struktur Molekul Asam Alkanoat dan Alkil Alkanoat 2. Tata Nama Senyawa Asam Alkanoat (Asam Karboksilat) Penamaan sistem IUPAC senyawa asam alkanoat mengikuti penamaan senyawa alkana di mana akhiran "a" diubah dengan "oat" dan ditambahkan kata "asam" di depannya.Penomoran dimulai dari atom C yang mengandung gugus fungsi asam alkanoat (COOH).

SOLUTION Kimia organik (alkil halida) part 1 Studypool
SolutionS. The alkyl group (CH 3 CH 2 CH 2 -) is a propyl group, and the halogen is bromine (Br). The common name is therefore propyl bromide. For the IUPAC name, the prefix for bromine (bromo) is combined with the name for a three-carbon chain (propane), preceded by a number identifying the carbon atom to which the Br atom is attached, so the IUPAC name is 1-bromopropane.

PPT GUGUS ALKIL sebagai cabang dengan rumus C n H 2n+1 PowerPoint Presentation ID5459599
Answer. 3.3: Alkyl Groups is shared under a CC BY-SA 4.0 license and was authored, remixed, and/or curated by Steven Farmer, Dietmar Kennepohl, Zachary Sharrett, Krista Cunningham, Tim Soderberg, William Reusch, & William Reusch. The IUPAC system requires first that we have names for simple unbranched chains, as noted above, and second that we.